This page explains why the pressure increases underwater and how to calculate it
This page is meant for non-divers who are reading a first time about underwater sports. The pages in the vademecum provide information about very well known theory to advanced rebreather knowledge. The pages are ranked on difficulty by means of an icon which makes it immediately clear whether it is basic information or more complex to high-tech backgrounds. Important is that the information from this vademecum can never replace a good rebreather training! If you have any additions or corrections, I would always be happy to receive them!
Calculating the underwater pressure is surprisingly simple when you round off the values. The pressure is the depth in meters divided by 10 plus 1 bar. So at 40 meters the pressure is 5 bar. Simple, isn’t it?
The question now is why is this so? That is already a lot more complicated. You can simplify that too by saying: the air above the water presses on the water with 1 bar. And every 10 meters deeper the pressure has increased by 1 bar. Think carefully about this formulation! This also means that in the intermediate depths the pressure increases as you go deeper. Example: the pressure at a depth of 36 meters is determined by the air column of 1 bar plus the water column of 36 meters which causes a pressure of 3.6 bar. Together this means 4.6 bar. At a depth of 36 meters there is a pressure of 4.6 bar.
This calculation is sufficiently accurate for diving calculations. It will be different if you dive in a special environment. Think for example of diving in a mountain lake at 2000 meters altitude. I will come back to that later on this page.
Let’s first have a look at the air column above us. I’m sure you’ve heard that planes fly in thin air. And also that balloonists used to bring an oxygen set to fly extremely high. Apparently the air is different in composition the higher you go and the pressure decreases with altitude. How about that?
Photo: Breathing in Irrespirable Atmospheres Robert H. Davis
Air pressure is also called atmospheric pressure. That is why pressure used to be expressed in atmosphere. Nowadays we express the pressure in bar. This is not entirely correct because we have agreements to express pressure in Pascal. But everyone knows the barometer and in diving we still express our pressure in bar at depth.
A bar is equal to a pressure of 1 kilo on a square centimeter. More official is 10 Newton / cm2. Actually we express ourselves in a value that is valid for each cm2. If there is a pressure of 6 bar, this means that at that place 6 kilos or 60 N presses on every centimeter.
Let’s now take a look at the International System of Units (SI system) which is 1 bar = 10,000 pascal. You can also say 1 bar = 10 kPa. With this knowledge we now look at a table in which the air pressure and altitude are shown we see the following:
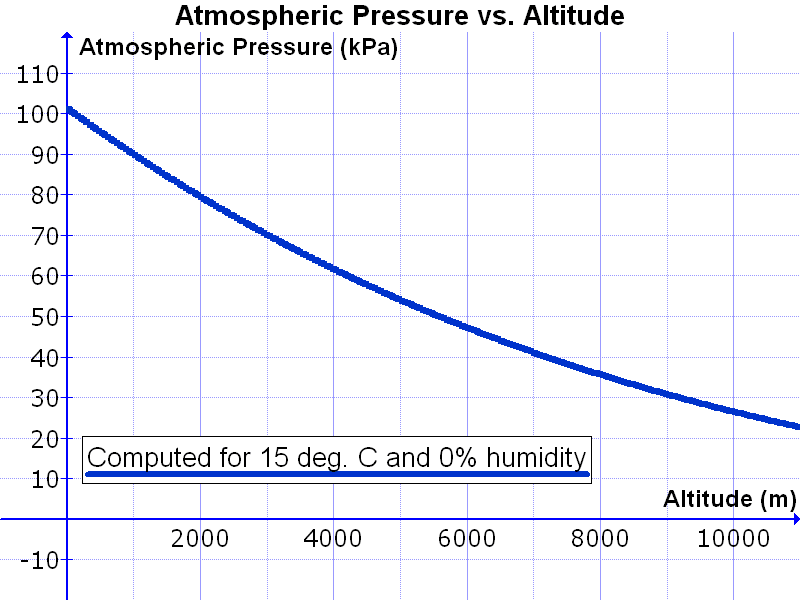
You can see that the air pressure depends on the temperature and humidity. If we choose those, we see that the air column that presses 1cm2 at 10,000 meters height presses about 0.28 kilos on that centimeter. At 400 meters height it is 0.6 kilo and at 0 meters it is 1 kilo.
So you can say that the air pressure decreases as you get higher but the relative decrease becomes smaller and smaller.
At 0 meters we speak of sea level, so at 15 degrees and the lack of moisture there is a pressure of 1 kilo on every square centimeter.
We also know the term Average sea-level which is set at 1013.25 mbar = 1.01325 bar. For more information about air pressure, an excellent page can be found on Wiki
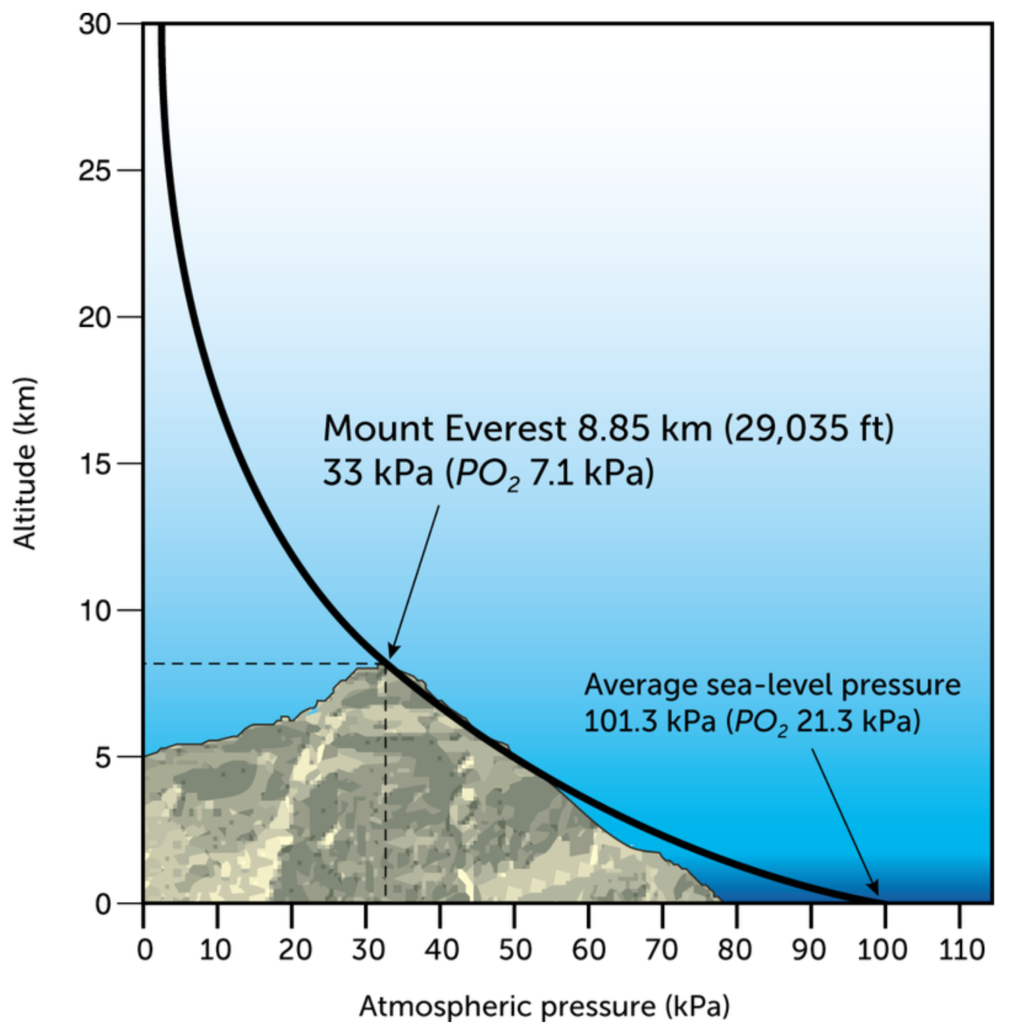
This image should now speak for itself
Let’s return to our watery environment. We now know that the air above the water at sea level exerts 1 bar of pressure. Before we go any further, first something about manometers. If you buy a manometer and look at it you see that the pointer is on 0-bar. Strange, because there was 1 bar pressure, right? We agreed that we only show the extra pressure, so the overpressure. That’s why you sometimes see in calculations bar.o, the .o stands for overpressure. We also know bar.a that means the absolute pressure so a pressure gauge with a scale bar.a should be at 1 bar. The bar.o is so common that when measuring absolute pressure we talk about vacuum manometers. These run from 0 to -1 bar.

Most people know that a liter of water weighs 1 kg. A liter is also a volume! A liter of water takes up a space of 10 x 10 x 10 cm. We also know that pressure is expressed in the weight that presses 1cm2. Sharing the cube is cubes of 1 x 1 x 1 cm we see the following:
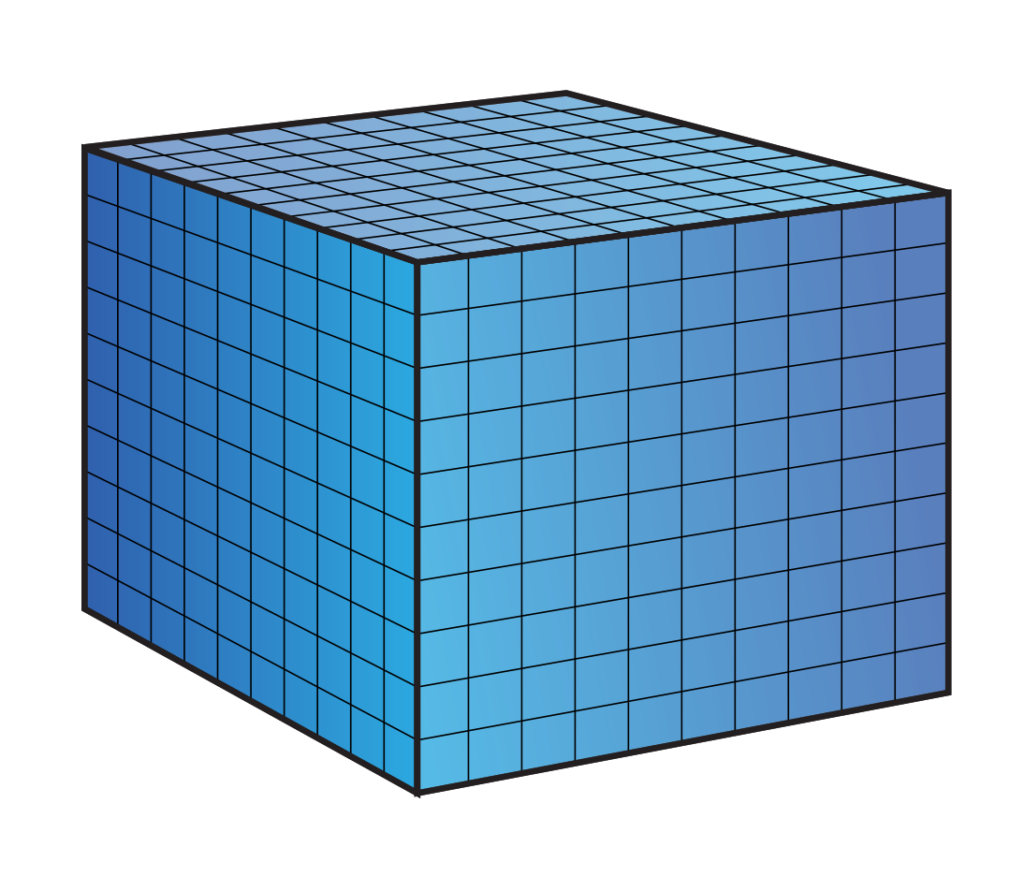
If we now look at the ground plane we see that it consists of 10 x 10 square centimeters, so 100 planes.
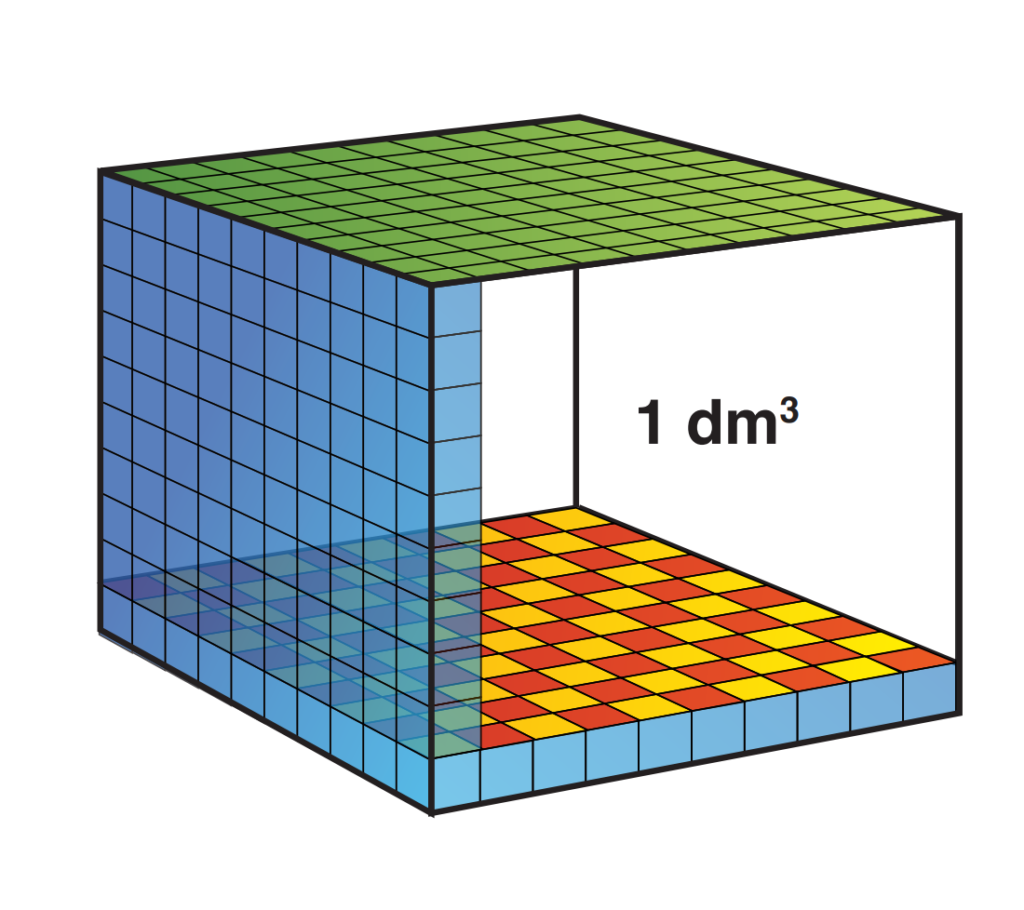
Now suppose we stack all blocks on top of each other. We then have the weight of 1 kilo of pressure at 1 cm2 and we know that this causes 1 bar of pressure. How high will the stack be? 100 stacks of 10 cm form a bar of 10 meters high (after all, 1000 cm = 10 meters). We have now established that a water column of 10 meters exerts a pressure of 1 bar! Together with the air pressure we now know that when I am at 10 meters deep under water I am under a pressure of 2 bar!
So now we can make a sum by making a formula:
the pressure on dive depth = the dive depth in meters divided by 10 plus 1 bar.
P = DD/10 + 1 bar
If we apply this to a dive depth of 20 meters, we now know that there is a pressure of 3 bar.
Same for 70 meters 8 bar and for 36 meters 4.6 bar.
We now also know that this depends on a lot of things and is actually a rounding off because a lot of things influence the exact outcome:
Humidity
Temperature
Altitude (sea level or mountain lake)
Fresh or salt water
Where do I measure, at my feet or on my wrist
Precision of the measuring instrument
For normal dive calculations only fresh or salt water is taken into account. Fresh water weighs 1000 grams per liter, seawater weighs an average of 1024 grams per liter. You will see in the calculations that the effect is small.


Imagine a cross-section of 800 cm2 at 90 meters, the weight on this suit will be………….

Therebreathersite was founded by Jan Willem Bech in 1999. After a diving career of many years, he decided to start technical diving in 1999. He immediately noticed that at that time there was almost no website that contained the history of closed breathing systems. The start for the website led to a huge collection that offered about 1,300 pages of information until 2019. In 2019, a fresh start was made with the website now freely available online for everyone. Therebreathersite is a source of information for divers, researchers, technicians and students. I hope you enjoy browsing the content!
