This page is about the relationship between pressure and volume
In 1627-1691 lived an Irish scientist who before diving drafted perhaps the most important law named after him, Boyle’s Law. Sometimes this law is also called Boyle-Mariotte law. The French physicist Edme Mariotte (1620–1684) discovered the same law independently of Boyle in 1679, but Boyle had already published it in 1662. Mariotte did, however, discover that air volume changes with temperature. Thus this law is sometimes referred to as Mariotte’s law or the Boyle–Mariotte law.

Robert Boyle (1627–1691), an Irish-born English scientist, was an early supporter of the scientific method and founder of modern chemistry. Boyle is known for his pioneering experiments on the physical properties of gases, his authorship of the Sceptical Chymist, his role in creating the Royal Society of London, and his philanthropy in the American colonies. In the painting, Boyle is shown in long curled wig, white neck cravat, and sleeves, and black coat; seated facing right with head turned three quarters left, looking out; turns page of book with left hand and gestures toward book with right; upholstered chair and tablecloth in silver brocade.
Boyle found the relationship between pressure and volume. Mariotte found that pressure and volume changed with temperature. If we combine the laws you can say that at a constant temperature the relation between pressure and volume remains constant. in the form of a law you write P = pressure and V = volume and C = Constant, is the law: P x V = C
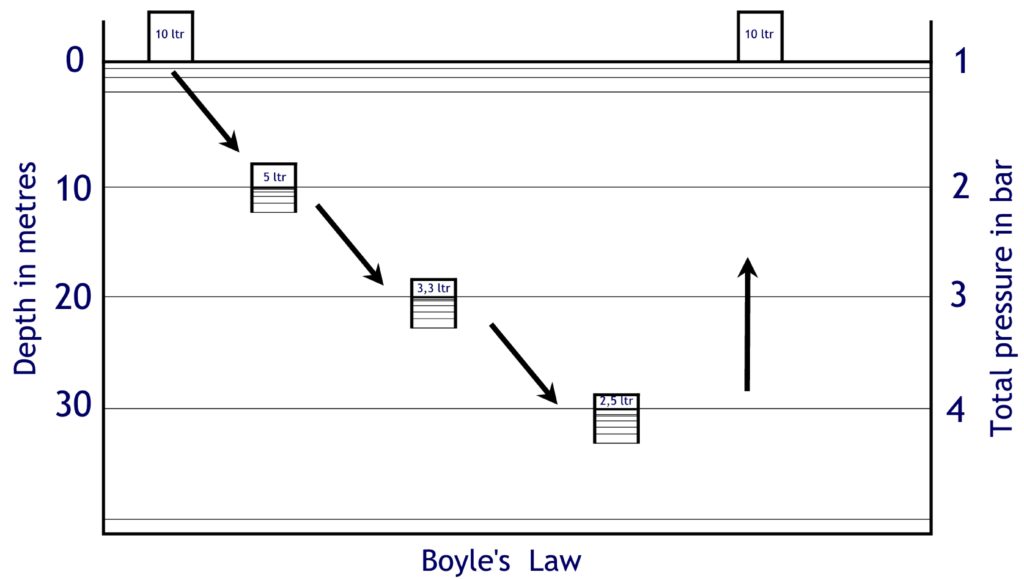
If we take a transparent bucket to a dive site and put it upside down on the surface of the water filled with air, in our example there is 10 litres of air in the bucket. Now we descend to a depth of 10 metres, we know that the pressure increases from 1 to 2 bar. A doubling. Boyle now says that if the pressure doubles, the volume must halve. The first sum was 10 litres x 1 bar = 10. Our constant is 10 because that’s the contents of the bucket and it doesn’t change. If we now go to a depth of 10 metres, the pressure becomes 2 bar. We can now calculate the volume because in the formula P x V = C we know the P and C are 2 x V = 10. From this it follows that the volume is 5 litres. So there is 5 litres of air of 2 bar in the bucket!
If we go another 10 metres deeper, at a depth of 20 metres there is an ambient pressure of 3 bar. The contents of the bucket (the constant) is still 10 litres. So the air in the bucket is compressed by 3 bar. P x V = 10 ; 3 x V = 10 gives 3.33 litres for the volume. So at a depth of 20 metres there are 3.33 litres of air of 3 bar in the bucket (and also 6.66 litres of water of 3 bar!).
At 30 metres there is 4 bar, there are now 2.5 litres of air in the bucket because 4 x 2.5 = 10.
Calculate for yourself how much air would be left in the bucket if the bucket were taken to a depth of 300 metres?
Question?


Why is this now so important to know? Boyle’s law plays a role in diving because it has to do with the following phenomena:
- Squeeze
- Why you must exhale during a descent
- Buoyancy underwater
- Decrease of volume of your diving suit
- Increasing use of gas with depth
- Volume of your gasvolume in scuba bottle
- Decreasing volume of counterlung from rebreather
For now this is enough about the relation between presure and volume. Next subject shows more information about the influence of the Temperature.

Therebreathersite was founded by Jan Willem Bech in 1999. After a diving career of many years, he decided to start technical diving in 1999. He immediately noticed that at that time there was almost no website that contained the history of closed breathing systems. The start for the website led to a huge collection that offered about 1,300 pages of information until 2019. In 2019, a fresh start was made with the website now freely available online for everyone. Therebreathersite is a source of information for divers, researchers, technicians and students. I hope you enjoy browsing the content!
