This page explains how the pressure of gases in a gas mixture develops underwater.
John Dalton (1766-1844) lived in England and was originally not encouraged to study by his church background. He was inspired by John Gough, a blind English philosopher of nature and experimentation who became known for his own research and the influence he had on John Dalton. At the age of 27 he was appointed teacher of mathematics and natural philosophy at the “New College” in Manchester, a dissenting academy (the lineal predecessor, following a number of changes of location, of Harris Manchester College, Oxford). He remained there until the age of 34, when the college’s worsening financial situation led him to resign his post and begin a new career as a private tutor in mathematics and natural philosophy. (Source WIkipedia)
Because we saw in an earlier explanation that the pressure of a gas increased under water because it was compressed, it is interesting to know what happens to the components in a gas mixture. We take an example by looking at normal breathing air.
Breathing air consists of a mixture of gases. Air that is normally inhaled under atmospheric conditions in our example has an air pressure of 1.013 bar. This is a normal average air pressure. The air consists of a number of important components of which Oxygen and Nitrogen are the most important for us as divers.
Besides Nitrogen and Oxygen, atmospheric air also contains other components. These components are separated from the air by manufacturers to be used for industrial purposes in a wide variety of processes. The relatively small proportion of these components is of only minor importance to recreational divers, but as we dive deeper these gases can have an influence. The composition of air for dry atmospheric air is as follows:
| Composition of dry atmospheric air of 1,013 bar expressed as a percentage fraction | |||
| Gas | Formula | Percentage % | |
| Nitrogen | N2 | 78,084 | |
| Oxygen | O2 | 20,964 | |
| Argon | AR | 0,9340 | |
| Carbon dioxide | CO2 | 0,04 | |
| Neon | NE | 0,001818 | |
| Helium | HE | 0,000524 | |
| Methane | CH4 | 0,000179 | |
Of course, the composition of air can be different if we compare clean mountain air with air from the centre of a large city.
The total pressure can also change because of the weather, or because we are at a different altitude.
If we now look at each component of the composition of the gas, it is Dalton who has found the formula and his law is described as follows:
Dalton’s law (also called Dalton’s law of partial pressures) states that:
In a mixture of non-reacting gases, the total pressure exerted is equal to the sum of the partial pressures of the individual gases.
When you read that for the first time it sounds quite complicated, but with the picture below it becomes clear as soon as possible:
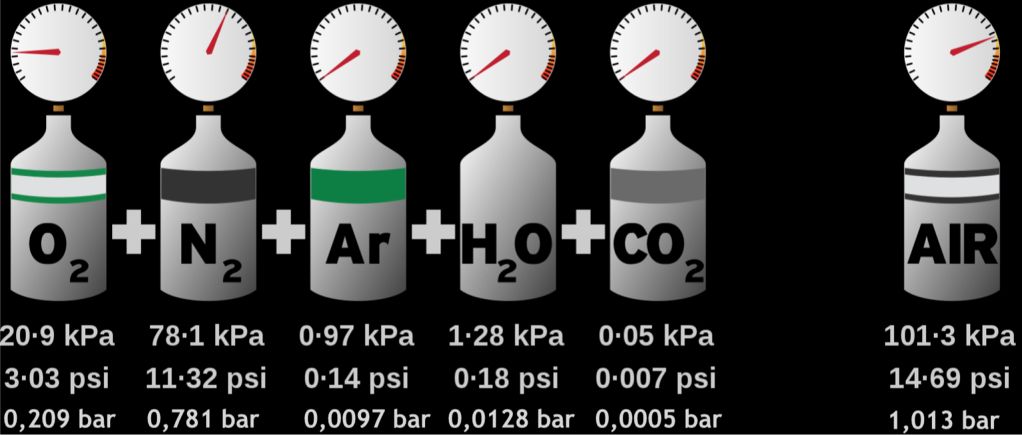
You can see that the pressure of the gas is the sum of the pressure of each component. Each component represents a volume share in the mixture. At the same temperature, this corresponds to what we call it: The partial gas pressure.
partial pressure = (total absolute pressure) × (volume fraction of gas component)
Example: The partial pressure of oxygen in our air mixture = 1.013 x (volume fraction or gas component)
But what is Volume fraction of a gas component? That means if oxygen is 20.9% of the mixture, then the volume fraction is 20.9 / 100 = 0.209.
We do not put unity behind a volume group. We are talking about the volume oxygen fraction is 0.209
Now we look again at the calculation of the partial pressure of oxygen we see:
partial pressure = (total absolute pressure) × (volume fraction of gas component)
So:
The partial pressure of oxygen in our example = 1.013 bar (air) x 0.209 bar (oxygen) = 0.21 bar
For nitrogen, the following applies: The Partial Pressure of Nitrogen in our example = 1.013 (air) x 0.781 bar (nitrogen) = 0.79 bar.
You will see that in the calculation we do for dives, these two values often recur. The other gases are so insignificant that they are left out of the calculations. Adding up the two partial pressures of Oxygen and Nitrogen, you will end up with 1 bar. We also use this value (1 bar) as a basis in our calculations. You now know that all these values have been rounded off.

©National Portrait Gallery, London
We now know that a breathing gas consists of components. Each component forms a part of the mixture and together they form the total pressure of the mixture. Dalton has described this in his law as follows:
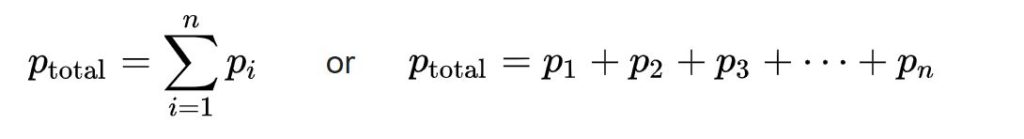
But what happens when we dive with our breathing gas? To take the gas with us, we put it into a compressor and compress it to a high pressure that can be carried in our diving cylinder. Below you can see a filling panel to which a compressor is connected that compresses atmospheric pressure from 1 bar to 230 bar.

We are now going to dive and take our gas supply with us in the diving cylinder. At a depth of 10 meters our regulator will lower the pressure in two steps to the ambient pressure. The first step is performed by the first stage, the part that is screwed onto the cylinder valve. This reduces the pressure to what we call the medium pressure. This pressure is often 10 bar + ambient pressure. At a depth of 10 meters there is an absolute pressure of 2 bar, so an air pressure of 12 bar will exist in the hose. The mouthpiece reduces this 12 bar to the ambient pressure of 2 bar. The diver therefore breathes breathing gas of 2 bar.

The diver breathes a gas of 2 bar at 10 meters. With our Dalton law we can now calculate the partial pressure of the gas components. The oxygen pressure is 2 bar x 0.21 = 0.42 bar. Nitrogen is 2 bar x 0.79 bar = 1.58 bar. We write pO2=0.42 and pN2=1.58. The total pressure is Ptot=2 bar. Filling in the partial pressures we see Ptot= pO2+pN2 and Total Gas pressure 2 bar = Oxygen pressure 0.42 bar + Nitrogen pressure 1.58 bar.
We now know what subtle pressure is and how to calculate it. A second example:
Question: What is the Partial Nitrogen Pressure of the breathing gas when a diver dives with compressed air at a depth of 40 metres?
Answer: Ptot = 40m/10 + 1 bar = 5 bar
Mixture is 21% Oxygen and 79% Nitrogen
The nitrogen fraction is 79
The volume fraction of Nitrogen is 0.79
The partial nitrogen pressure at depth is 5bar x 0.79 = 3.95 bar so pN2=3.95 bar
These examples are about normal breathing air. We can do the same calculation for Nitrox mixtures. Nitrox mixtures are mixtures with an increased oxygen content. Imagine diving with Nitrox 36, a widely used mixture for longer dives. The oxygen fraction in this mixture is 36 %. If we now ask the same question, what is the partial nitrogen pressure at 40 metres, we can calculate it as follows:
Answer: Ptot = 40m/10 + 1 bar = 5 bar
Mixture is 36% Oxygen and 64% Nitrogen
The nitrogen fraction is 64%
The volume fraction of Nitrogen is 0.64
The partial nitrogen pressure at depth is 5 bar x 0.64 = 3.2 bar so pN2=3.2 bar
In the previous example we saw that by increasing the proportion of oxygen in the respiratory gas we obtained a lower pN2. So it seems attractive to maximise the oxygen share because the Nitrogen part is responsible for the time we can stay underwater. The less nitrogen is absorbed in the blood the longer we can dive. Unfortunately there is a factor that throws a spanner in the works, namely the partial oxygen pressure. This is subject to limits. Oxygen becomes toxic at a certain partial pressure and causes a condition called Hyperoxia. The partial pressure when diving to 40 metres with Nitrox 36 results in a partial oxygen pressure of oxygen:
Answer: Ptot = 40m/10 + 1 bar = 5 bar
Mixture is 36% oxygen
The oxygen fraction is 36%
The volume fraction of oxygen is 0.36
The partial nitrogen pressure at depth is 5 bar x 0.36 = 1.8 bar so pO2=1.8 bar
DALTON: Ptot = pO2+pN2 = Ptot = 3.2 bar N2 + 1.8 bar O2 = 5
In the next vademecum page we will look in more detail at the limits we link to oxygen pressure and nitrogen pressure and get to know the limits of compressed air mixtures.

Therebreathersite was founded by Jan Willem Bech in 1999. After a diving career of many years, he decided to start technical diving in 1999. He immediately noticed that at that time there was almost no website that contained the history of closed breathing systems. The start for the website led to a huge collection that offered about 1,300 pages of information until 2019. In 2019, a fresh start was made with the website now freely available online for everyone. Therebreathersite is a source of information for divers, researchers, technicians and students. I hope you enjoy browsing the content!
