Oxygen as a breathing gas
Oxygen is a colorless, odorless, tasteless gas.

Oxygen was discovered in 1771 by the Swedish pharmacist Carl Wilhelm Scheele. The rediscovery by Joseph Priestley only made it more widely known. It was soon understood that this gas, although only one-fifth of the air on our planet, makes combustion possible, as well as the breathing of humans and animals (and plants, during the darkness).

It was Antoine Lavoisier who gave it its scientific name oxygenium (acidifier). It was thought that the element was an indispensable component of an acid.

The fact that the electrolysis of a large number of acids (with hydrogen halides as an exception) produced oxygen gas at the cathode was particularly responsible for this conviction. Although oxides of many elements are acid-forming, the reverse is not true: to form an acid, oxygen is not necessary. The formation of hydrogen chloride from hydrogen gas and chlorine gas is an example of this.
For use in the diving world, Oxygen is the most important gas next to Nitrogen. Oxygen is essential for life. Oxygen is a very important fuel for your body cells. You breathe it in through your lungs, where it is absorbed into the blood. There it binds to the haemoglobin in the red blood cell. The red blood cells flow through the whole body and release oxygen to all body cells along the way. In this way your organs such as the brain, kidneys, liver or muscles get their energy.
Oxygen is formed on earth by photosynthesis. In this process plants and algae use their chlorophyll grains to capture light energy and convert water and carbon dioxide into sugar and oxygen. Part of the sugar is broken down and converted into energy, while the rest is stored for growth.

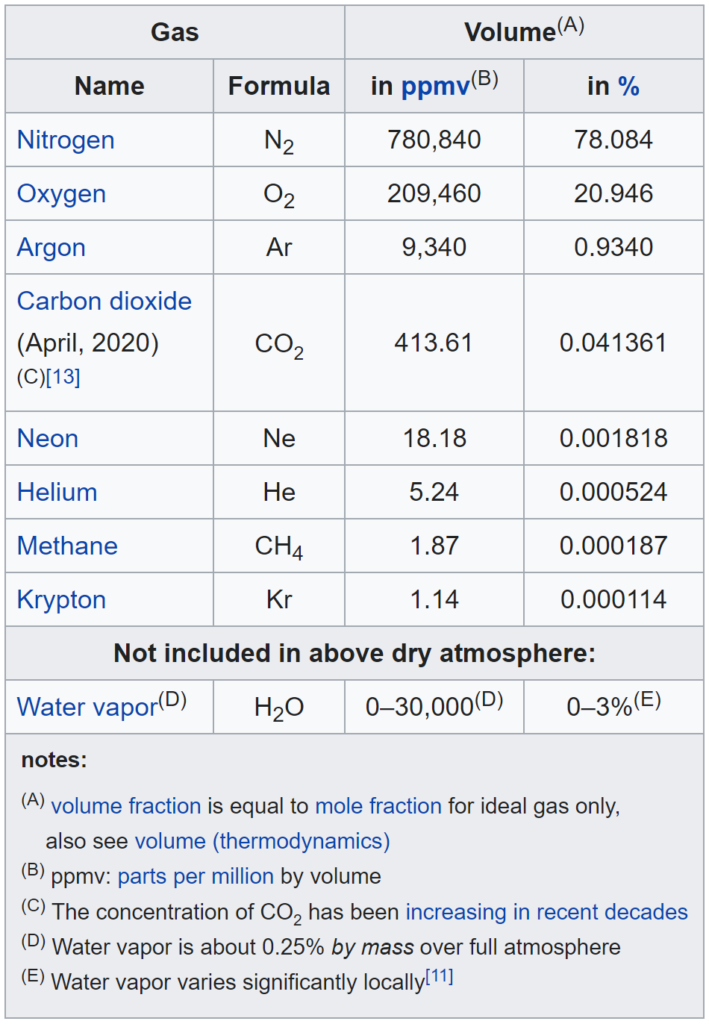
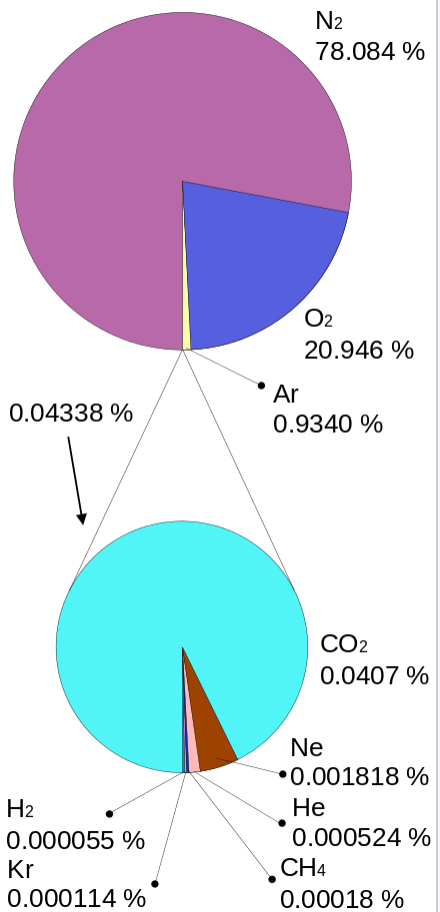
Oxygen is one of the most important components in the composition of air. Nitrogen takes up more than ¾ of the volume. Normal dry breathing air in the atmosphere contains the following elements:
Oxygen is a crucial component in diving. In dives with open circuit breathing systems normal breathing air, nitrox and trimix are used.
Opencircuit diving
Standard breathing air contains 21% oxygen. Nitrox is used regularly with an increased percentage of oxygen between 21 – 40%. Higher mixtures are also known as Nitrox but are also referred to as decompression gas. More oxygen in the mixture means that we can breathe it less deep (more later). Less oxygen means that we can also use the gas deeper. Trimix is a mixture of oxygen, nitrogen and helium. By replacing oxygen with helium, mixtures can be made that are breathed at depth and sometimes contain as little as 4% oxygen.
Further on this page it is described which limits apply to the gases in order not to cause toxicity.
Semi closed diving systems
With a semi-closed diving system, part of the gas used is reused and part is returned to the environment. In recreational applications, the systems are usually fed with gases containing between 21 and 60% oxygen.
Closed circuit breathing systems
Closed breathing systems work with all mixtures between 3 and 100% oxygen. The oxygen fraction is determined by the depth. Here the deeper the lower the fraction. Through a variable fraction in a closed system, a fixed partial pressure of the gas is realised. Nitrogen and helium are added as a diluted gas. The composition of the dilution gas is crucial for determining the maximum diving depth.
Oxygen as the most important life-sustaining gas, but also a limiting factor!
Without oxygen, a diver cannot survive underwater. Diving systems therefore always provide the diver with the right amount of oxygen and the right partial pressure of oxygen.
What are the limits when breathing oxygen?
Volume:
A human being has a metabolic consumption. This consumption is linked to labour.
| Zero exertion | 0,3 litres/min |
| Rest | 0,5 litres/min |
| Moderate work | 0,9 litres/min |
| Heavy work | 2,0 litres/min |
| Extreme work | 3,0 litres/min |
Pressure:
When oxygen is breathed in a normal environment, the gas consists of 21% Oxygen. We can increase the oxygen content up to 100%. Patients who need extra oxygen sometimes breathe 100% oxygen. This mixture has a partial pressure of 1.0 bar (atmospheric) at an ambient pressure of 1 bar. Higher partial pressures can only be achieved when the ambient pressure rises. This is the case under water. If we were to breathe pure oxygen at a depth of 10 metres, we would inhale a partial oxygen pressure of 2.0 bar. A human being can breathe a higher partial oxygen pressure but there are limits to this. The subject has been the subject of research and discussion for years. If a person breathes too high an oxygen pressure hyperoxia can occur. Hyperoxia is an acute oxygen poisoning.

Acute oxygen toxicity (Paul Bert Effect)affects the central nervous system causing muscular twitching, dizziness, nausea and vomiting. Also there can be acoustical effects like ringing in the ears. Finally it can cause vomiting, convulsions and coma. This usually does not occur below 1,8 bar in recompression tanks or below 1,6 for immersions. For safety reasons within the diving community it is a common vue to breathe oxygen with a maximum of 1.3 – 1.4 bar during longer periods and maximum 1.6 during decompression (100% oxygen @ 6 mtrs of depth).
Another type of oxygen toxicity is pulmanory oxygen toxicity(Lorrain Smith Effect). This is caused by breathing oxygen above 50% during longer periods in normal atmospheric conditions. Higher concentrations (80-100%) breathed for more than 8 hours can cause irritation of the airways, cough and feelings of shortness of breath. Longer exposure for greater than 24 hours can cause lung damage.


Prof. Kenneth Donald did a lot of research on oxygen poisoning in divers in the 40s. His book Oxygen and the Diver is a phenomenal source to gain insight into the complex matter of oxygen poisoning.
Fysical Properties of Oxygen
Oxygen is a gas in normal atmospheric conditions (15°C and 760 mm Hg). Oxygen is indispensable for the maintenance of life and for combustion. It is colorless, odorless and tasteless. It accounts for 20,94 percent by volume of the air we breathe. At atmospheric pressure at temperatures below
-183°C, it is pale blue liquid, slightly heavier than water. Oxygen is a highly reactive gas which combines directly with most elements to form oxides in accordance with temperature conditions. Certain elements like phosphorus and magnesium ignite spontaneously in oxygen (or air), while noble metals are only slowly oxidized at very high temperatures.

Oxygen is indispensable for combustion. It is liable to react violently with organic substances. Oxygen is shipped either as a gas under high pressure in cylinders, or as a liquid at low temperature under its own vapor pressure.
Formula: O2
Atomic number: Z=8
Molecular weight: 31.9988 g.mole-1
Molecular dimensions: 4,2-2,8 Å
First ionization potential: 12.059 eV
Gas density: 1,4289 kg.m-3
Biological properties of Oxygen
Oxygen is the gas wich is essential for sustaining life. It accounts for 20,95% by volume of the air we breathe, the remaining gas consists of nitrogen, argon (see above). Oxygen-poor atmospheres (less than 17% oxygen by volume) cause serious damage, capable of leading to death by asphyxiation if the oxygen content becomes excessively low (less than 12% by volume). The dangers of under-oxygenation are described in the nitrogen pages of my website.
Contrary to widespread belief, an oxygen-poor atmosphere does not cause respiratory difficulty or a feeling of suffocation in a healthy person, but is only manifested by minor effects, substantially corresponding to periods of incipient anesthesia (balancing problem, dizziness etc.). If the atmosphere contains less than 7% oxygen by volume, asphyxiation is extremely rapid, without warning symptoms.
On the other hand, at atmospheric pressure, it is possible to breathe atmospheres containing up to 75% oxygen by volume without any danger to the organism (but with high risks of inflammation of organic matter). Above 50% oxygen by volume a form of oxygen toxicity is pulmanory oxygen toxicity(Lorrain Smith Effect). This is caused by breathing oxygen above 50% during longer periods in normal atmospheric conditions. Higher concentrations (80-100%) breathed for more than 8 hours can cause irritation of the airways, cough and feelings of shortness of breath. Longer exposure for greater than 24 hours can cause lung damage.
Oxygen fires in diving

Oxygen is notorious for the many fires that have occurred in diving systems. Because oxygen is part of the fire triangle, fires always require ignition and fuel. Oxygen itself is the catalyst, say the engine that starts the fire. Because in oxygen systems almost all materials become flammable at high pressure, ignition is often the critical factor! That metal can burn is hard for many divers to understand, until the comparison is made with a cutting torch. After all, everything is present there, namely oxygen, fuel and an ignition (flame).
In a high-pressure oxygen environment, everything becomes flammable. The choice of material is therefore essential. There are lists of the flammability of materials in relation to pressure. For example, magnesium can spontaneously ignite in an oxygen environment of 1 bar, stainless steel only requires 8 bar of pressure and brass only burns above 600 bar. It is therefore very important to carefully select the materials to be used in an oxygen system. The thickness of the material also plays a role. Everyone is familiar with the phenomenon that steel wool easily burns in a normal atmospheric environment. We also call this the distribution. Powdered materials burn easily. As wall thicknesses increase, materials become less flammable.
The effect of the oxygen in the combustion triangle is largely determined by the oxygen pressure. The higher the oxygen pressure, the higher the risk of fire.
We have many mechanisms that can trigger an ignition. The most important ones for us divers are heat through compression and particle impact. Heat through compression occurs when valves are opened too quickly. The gas will then be stopped at the next shut-off, causing an enormous increase in temperature on site. If this happens too quickly and the materials are flammable at the created temperature, an ignition will take place resulting in fire. If a cylinder with 200 bar is opened at a speed of < 1 second, the temperature can increase to above 1000 gr Celsius. High pressure hoses often have a PTFE (teflon) lining which ignites around 430 degrees Celsius. It is therefore essential to open valves with enriched mixtures as slowly as possible.
Another fire mechanism is particle impact. Small metal particles or contaminants in an oxygen system can fly through the gas stream at high speeds through the system. As soon as they collide, energy is released which can lead to ignition. Therefore it is essential to keep oxygen systems (cylinders, hoses etc.) in oxygen service.
A list of the most common ignition mechanisms is given below:
• Particle impact
• Heat of compression
• Flow friction
• Mechanical impact
• Friction
• Fresh metal exposure
• Static discharge
• Electrical arc
• Chemical reaction
• Thermal runaway
• Resonance
• External heat
Hydrocarbons and organic contaminants
Many moving parts are lubricated. Threads in pressure systems are often secured with threat sealants. Divers touch parts that come into contact with oxygen with bare hands. All these actions lead to contamination. Oxygen systems should be perfectly clean. There are various methods for this which require specific knowledge in order to clean them correctly.
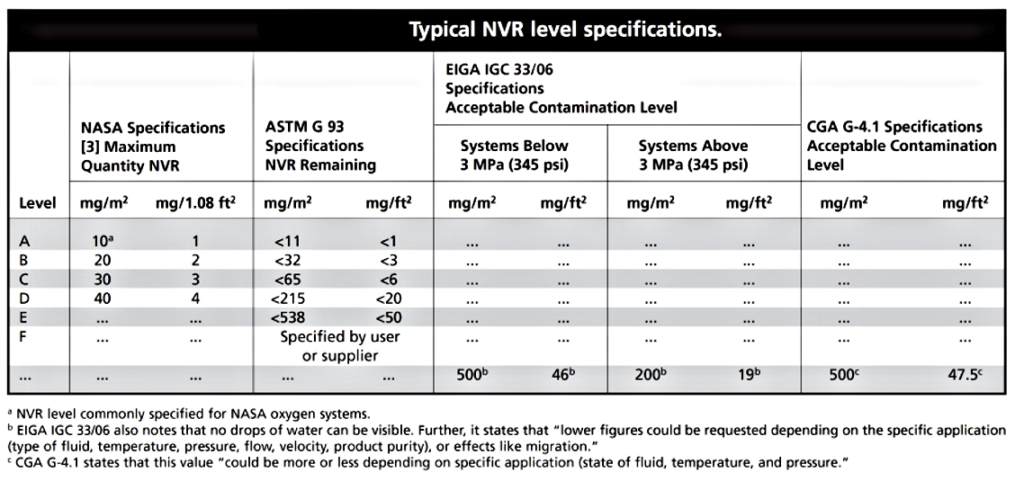
It has been shown that oil films in the range of (27 to 70 mg/m2 are vulnerable to ignition by heat of compression, and that as little as 10 mg of particulate has ignited components. The level of contamination necessary to markedly increase the ignition hazard has not been established.
Therefore, a good practice is to be conservative by specifying a cleanliness level equal to or better than the level experience has shown to be acceptable for the application. Cleanliness levels typically are specified with a number and letter, such as 100A. The letter corresponds to the allowable
level of nonvolatile residue (NVR), which gives an indication of the amount of oils, greases, and hydrocarbons present on the parts. As shown in table below, “Level A” corresponds to several different amounts of allowable NVR, depending on what cleaning specification is used. Therefore, it must be ensured that the desired level of NVR is obtained by paying close attention to what specification is used. The number corresponds to the maximum allowable particle size, and for any
given level there is a distribution allowed according to the size of the particulate, as shown in Table
Typical NASA, ASTM, and CGA cleanliness levels are given in Tables 6-1 and 6-2. The maximum allowable NVR on parts used for oxygen service is normally 10 mg/m2 for aerospace applications and ~538 mg/m2 for industrial applications. Particulate requirements for specific components
and systems depend on the application; levels 50, 100, and 300 are most common. In some cases, cleanliness requirements may be loosened for low-pressure systems.
Good habits
For divers and medical staff using oxygen mixtures > 21% the following good habits apply:
- Keeping systems > 21% separate from compressed air systems using normal breathing air
- Always analyse and label mixtures
- Keeping oxygen systems clean and inspecting and cleaning them at least once a year
- In all cases, the increase in pressure should take place as slowly as possible.
- Use only oxygen suitable for human respiration
- Be apprehensive of oxygen fires, ensure good ventilation
- Filling systems must be earthed to prevent static discharge
- Personnel operating oxygen equipment must have been trained for this purpose.
- Personnel should be instructed in the assembly of pressure regulators on cylinders.
- Personnel must be aware of the hazards and risks associated with the use of oxygen equipment.
- Check the markings on the cylinder and test stickers on the pressure regulators to determine if the components may be used.
- The use of reducing devices made of copper alloys such as brass or bronze is strongly recommended given the number of incidents with aluminium pressure regulators. Therefore, do not use aluminium reducing devices, partly because the aluminium when it is melted has a very high combustion energy and melts easily(applies to titanium pressure regulators!)
- A good reducing valve is equipped with an inlet filter made of an oxygen suitable material such as a filter made of (sinter) bronze.
- Check the regulator and cylinder before use (apply: LMRA= last minute risk analysis)
- After the manual removal of a plastic protective cap, the connection opening of the cylinder must always be inspected for contaminants.
- When installing a pressure reducing valve, never lay it on the ground or touch the thread with your fingers. This is to prevent contamination.
- The connection between the cylinder and the pressure reducing valve must always be clean and free of grease.
- Make sure that the cylinder is positioned vertically when starting up and that the opening of the operator’s tap is rejected. The valve should then be opened slowly and immediately closed again before the regulator is connected. The aim is to blow any contaminants out of the opening of the bottle valve.
- Connect the reducing valve after it has been established that the connection is intended for the bottle.
- Before opening the valve, set the flow meter to ‘0’.
- Pressure should always build up (very) slowly.
- If a leak is observed, the valve must be closed, the system must be vented and inspected.
- Never try to tighten the connection more under pressure in order to remedy the leak.
- Make sure that oxygen systems (bottles and regulators) are stored in a cool room in which no dirt, oil or grease traces can be found.
- Make sure that the room in which oxygen cylinders are filled and oxygen equipment is maintained is sealed. Make sure the room is clean and free of dirt, oil and grease
- Oxygen cylinders should preferably be stored in a vertical position.
- Smoking is prohibited in rooms where oxygen or oxygen mixtures are used.
- Place ‘no smoking’ warning pictograms inside and outside the room where oxygen is permanently used, stored or administered.
- Do not use open flames in the vicinity of an oxygen supply area.
- Do not allow smoking by other persons in the vicinity of a patient/victim.
- Ensure clean hands before working with the regulator and cylinder. This is especially in view of the risk of ignition by grease or oil, but also avoid contact with disinfecting or cleaning alcohol gels.
- Oxygen cylinders with a mounted pressure valve should not be worn on the tap or regulator. Carry the set by holding the bottle in your hands.
- Always close the tap on the oxygen cylinder after use.
- -Handle oxygen cylinders with care. If they fall, they should be presented for inspection.
- Oxygen cylinders with integrated pressure regulators should always be presented for maintenance after they have fallen.
- -Make sure that oxygen cylinders taken as a spare are full and check.
- -Ensure that oxygen cylinders are secured and cannot fall during transport in cars, on beds or other mobile applications.
- Store oxygen cylinders in a ventilated area. If Bull-nose connections are used, extra checks should be made to ensure that the regulator is connected to medicinal oxygen.
- If fibreglass-reinforced lightweight cylinders are used, be careful with sharp or scratching objects. These may damage the cylinder.
- -Before entering a magnetic MRI environment, the suitability of the oxygen set should be determined.
- Small oxygen cylinders are often fitted with a plastic sealing cap. This can be removed and replaced by hand. They do not guarantee the filling of a cylinder.
- Replace the o-ring or other seal with nitrile gloves. Do not touch the O-ring with bare fingers. The O-ring should be in oxygen service and packaged as such.
- Provide a clean, grease-free environment without bandages, textiles or other flammable products in the area where bottles are placed during transport.
- Oxygen cases must be internally clean and provided with suitable gloves and spare seals.
- Oxygen cylinders are nowadays also available with an integrated reducing valve. These make the installation of the regulator unnecessary. It is generally not possible to refill these cylinders yourself.
- Oxygen equipment must be provided with CE mark
- Oxygen equipment for medical applications must comply with Medical Device Directive 93/42/EEC (for equipment produced after 1998).
- Never place oxygen systems under a covering blanket or clothing.
- Clothing saturated with oxygen should be adequately ventilated for at least 15 minutes. For blankets or bedding, it takes 30 minutes before it is safe to approach a source of ignition.

Therebreathersite was founded by Jan Willem Bech in 1999. After a diving career of many years, he decided to start technical diving in 1999. He immediately noticed that at that time there was almost no website that contained the history of closed breathing systems. The start for the website led to a huge collection that offered about 1,300 pages of information until 2019. In 2019, a fresh start was made with the website now freely available online for everyone. Therebreathersite is a source of information for divers, researchers, technicians and students. I hope you enjoy browsing the content!
