
Henry’s law is a chemical law, named after the English scientist William Henry (1774-1836), which applies to the equilibrium situation of a solvent in contact with a gas. If there is contact between a gas and a liquid solvent, gas molecules will dissolve in the solvent. If this continues long enough, equilibrium will be reached, with an equal number of particles entering or leaving the solution. At such an equilibrium, the concentration of the dissolved substance is directly proportional to the concentration of the gas. This is called Henry’s law.
William was born the son of Thomas Henry, a pharmacist, in Manchester. Although Henry successfully studied medicine, starting in 1795 and obtaining his doctorate in 1807, he was unable to practise medicine due to his poor health. This gave Henry time to devote himself to research in chemistry, in particular on gases.
During his training, in 1803, he was already able to get a paper published in the Philosophical Transactions of the Royal Society about the amount of gases that could dissolve in water under varying pressure. His other articles deal with the composition of certain gas mixtures, the structure of hydrogen chloride gas (hydrochloric acid, HCl) and ammonia gas (NH3), and the disinfective potential of heat. In 1808 he was awarded the Copley Medal.
Source: wikipedia
Henry’s Law
Gas absorption is governed by Henry’s Law. that states that the amount of a gas that will be dissolved in a liquid at a given temperature is almost directly proportional to the partial pressure of that gas. The term “amount” refers to the number of molecules or mass of the gas. When gas is in solution, its actual volume is negligible and there is no volumetric increase in the amount of liquid. Henry‘s Law simply expresses the effect of partial pressure on the amount of gas that will dissolve in a liquid. Solubility is also dependent on temperature and the type of liquid. For example, the solubility of nitrogen in oil or fat is about five times its solubility in water at the same pressure. The lower the temperature, the higher the solubility. This explains why a warm bottle of carbonated beverage forms bubbles more actively than a cold one.
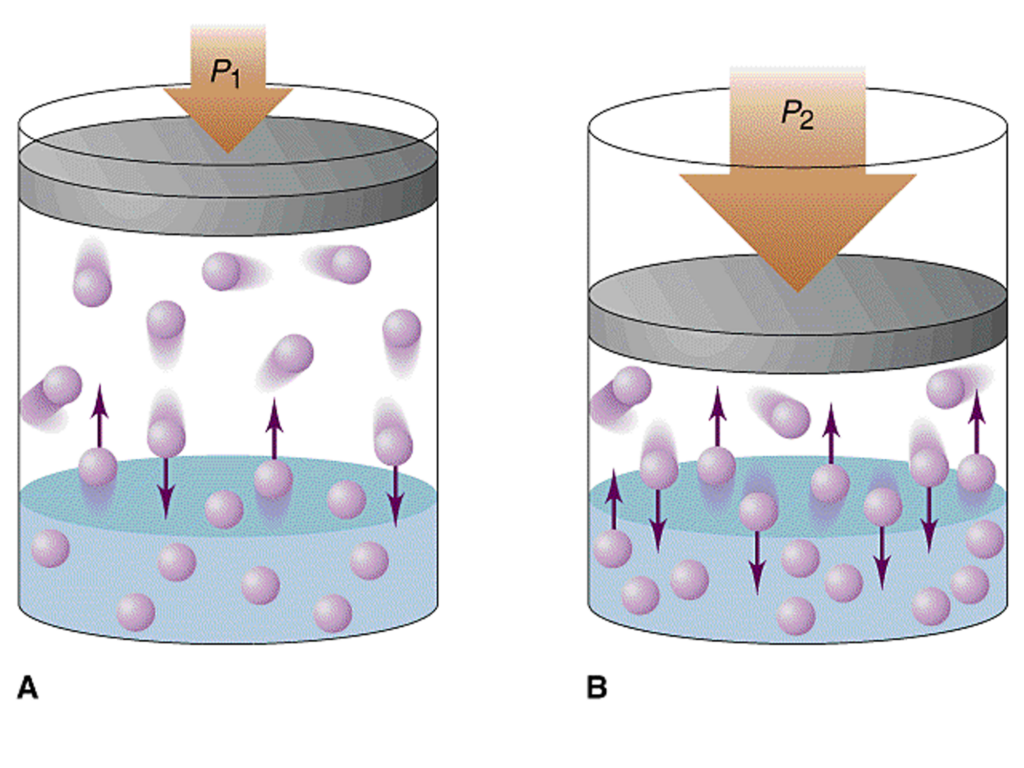
Gas diffusion refers to the intermingling of gas molecules. In diving‚ Henry’s and Dalton’s Laws are considered when dealing with the diffusion of gas in the human body under pressure. The difference between the partial pressure (or tension) of a gas inside of a liquid (or container) and its outside partial pressure will cause the gas to diffuse in or out of the liquid and control the rate of diffusion. This pressure differential is frequently called the gradient. If a gas-free liquid is exposed to a gas, the inward gradient is high and the rate at which gas molecules will migrate into the liquid is high. As the gas tension in the liquid increases, the rate of diffusion decreases and eventually reaches an equilibrium, where the gas tensions in the liquid and outside the liquid are equal. The liquid is then considered saturated for a given pressure and gas. The subjects of gas solubility and diffusion are important in the study of decompression sickness and nitrogen narcosis.
Textsource: Technical Diving from the Bottom Up, Kevin Gurr 2010 ISBN 978-0-9565849-0-8
published with permission.
Mathematically, Henry’s Law can be expressed as:
C = kP
Where C is the concentration of the gas in the liquid, k is the Henry’s Law constant (a constant that depends on the temperature and pressure), and P is the partial pressure of the gas above the liquid.
This original PDF of Henry’s Article 1802 could only published with the permission of The Royal Society
