Diving and behavior of gases
First Gas Law
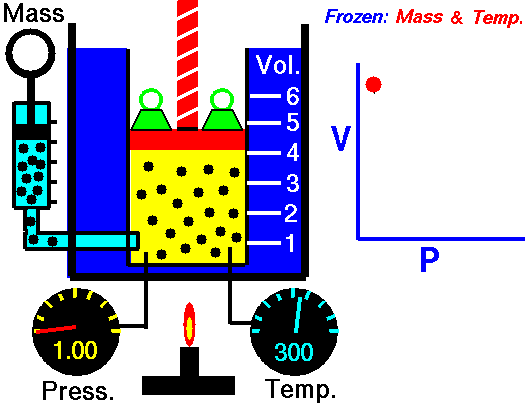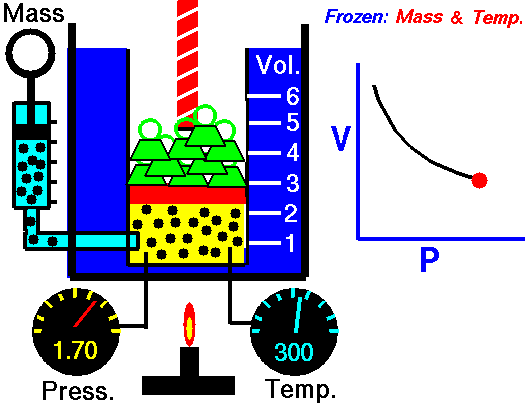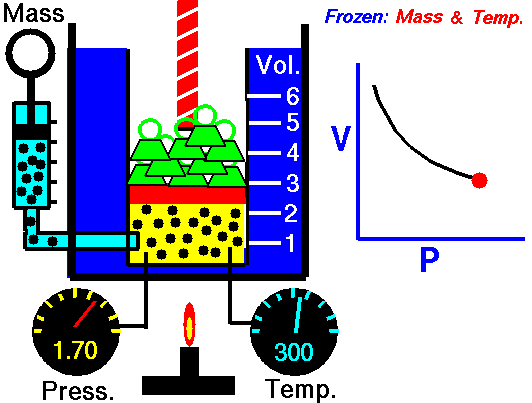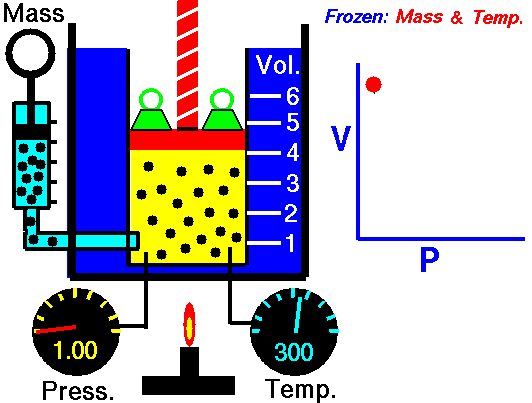
Robert Boyle discovered as early as 1662 that the pressure and volume of a gas had a constant relationship. His invention is also called the first gas law. French physicist Edme Mariotte (1620-1684) discovered the same law independently of Boyle in 1679, but Boyle had already published it in 1662. Mariotte did, however, discover that the volume of air changes with temperature. Therefore, this law is sometimes referred to as Mariotte’s law or Boyle-Mariotte’s law.
PxV=C
Robert Boyle was born into a wealthy and noble Irish family. He was the 7th son of Richard Boyle, Earl of Cork (in) and his wife Catherine Fenton Boyle . After studying at the University of Eton, he traveled through Europe from 1639 to 1644 and learned about the work of Galileo in Florence in 1641. Thus he learned the purely experimental method that characterized his entire scientific life. Master of a considerable fortune, he devoted it to the advancement of the natural sciences . Back in England he settled in Oxford in 1654 . He met Robert Hooke , a famous physicist who helped him build an air pump that Boyle needed for gas research. Thus he debated with Thomas Hobbes about the existence of a vacuum .
From 1645 he participated in a learned and benevolent society which he called in his letters the ” invisible college ” , but of which he mentioned neither the names of the members nor the activities, and which gave rise to various speculations.( in particular as a precursor group of the Royal Society ).
To him we owe the foundation of the Royal Society at a meeting on November 28, 1660 at Gresham College . The decision to create a Royal Society is confirmed by a first charter of King Charles II dated July 15, 1662, then in a second charter dated April 22, 1663, it becomes the Royal Society of London for the improvement of natural knowledge .
In 1663 he was elected ” fellow ” and in 1680 he was elected president, but he refused this honor and Christopher Wren was elected in his place.
A bequest from Robert Boyle made possible the establishment in 1700 of the Boyle School , in the grounds of Bolton Priory , North Yorkshire.

Second Gas Law
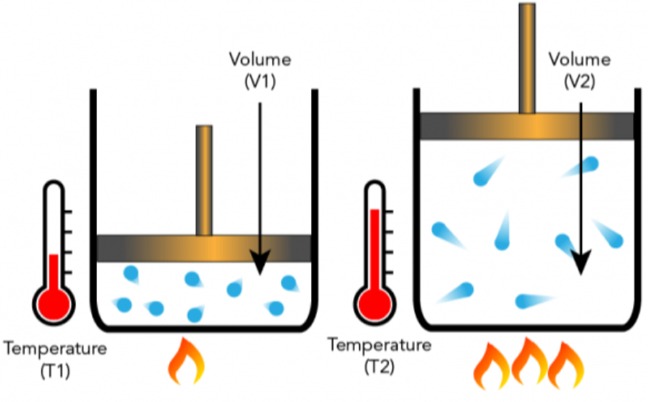
The second gas law Jacques Charles’s law (1780, also known as the law of volumes) is an experimental gas law that describes how gases tend to expand when heated.
V/T = C

Charles’s law is an experimental gas law that describes how gases tend to expand when heated. A modern explanation of Charles’ law is: If the pressure on a sample of a dry gas is kept constant, the Kelvin temperature and volume will be directly proportional.
Charles is best known for his flight in a hydrogen balloon called the “Charlière” ten days after the Montgolfier brothers made their first manned flight. On December 1, 1783, at two in the afternoon, the temperature was 4° C. Cheered on by more than 200,000 spectators, the balloonists Charles and Marie-Noël Roberts took off from an avenue in the Tuilerien of Paris. Champagne and wool blankets accompanied them in the wicker basket. The flight lasted two hours; there was a nippy cold, but hardly any wind. They rose to 1,800 feet (550 m) and landed nine miles outside Paris. Charles’ fellow passenger got out, and when the peasants, who were holding the contraption, let go, Prof. Charles ascended alone to an altitude of 3,000 feet in ten minutes. He got pain in his ears, got scared, but enjoyed the evening light and – as agreed – came down again after 35 minutes.[2] Charles was thus the first human to see the sun set twice, but never made another flight.

Third Gas Law
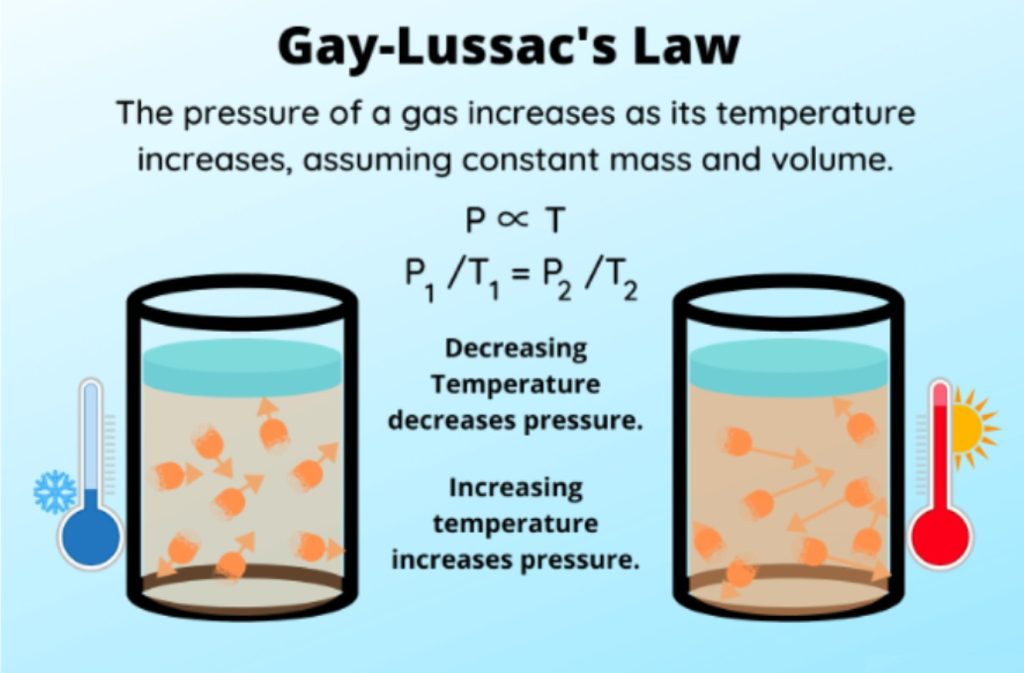
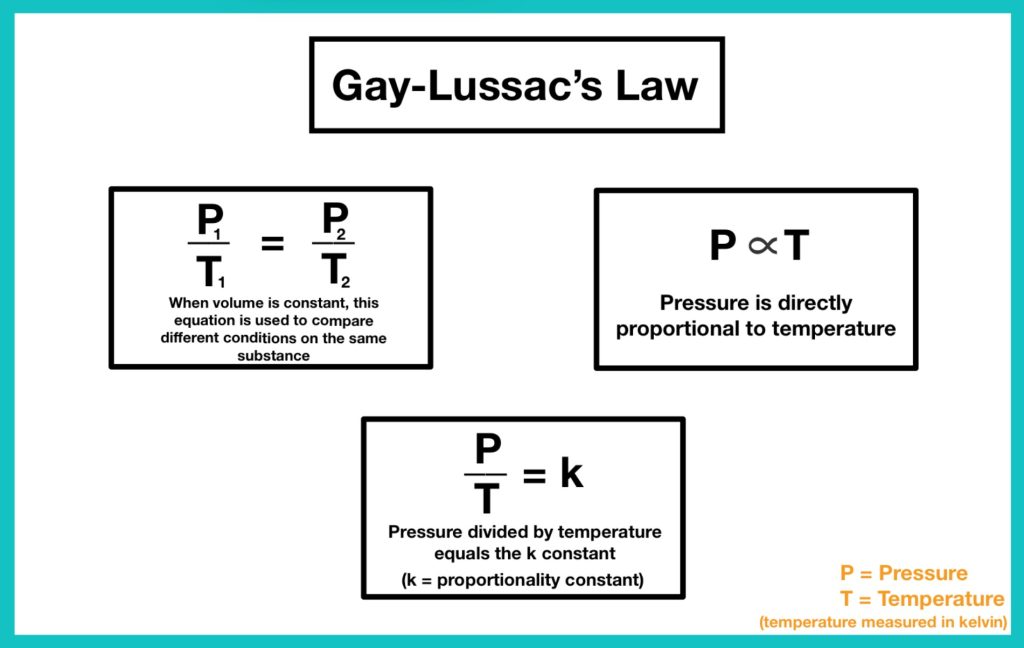
Joseph Louis Guy-Lussac’s third gas law was published in 1808 in which he stated that The pressure of a gas of fixed mass and volume is directly proportional to the absolute temperature of the gas.
P/T = C
Louis Gay-Lussac became professor of physics at the Sorbonne in 1808, professor of chemistry at the École polytechnique in Paris in 1809. In 1832 he became professor of chemistry at the Jardin des Plantes. In 1805, he and Alexander von Humboldt observed that two volumes of hydrogen and one volume of oxygen combine to form two volumes of water vapor. Later he found that similar simple ratios can be observed at the junction of all gases. This property is still known today as Gay-Lussac’s gas laws. Gay-Lussac also made important discoveries in the field of theoretical chemistry later, including those about potash, chlorine and iodine. Gay-Lussac was inducted into the exclusive Order “Pour le Mérite” in 1842. He is one of the 72 Frenchmen whose names are engraved in relief on the Eiffel Tower.

General Gas Law
These three laws form the basis of how gas behaves at a variable pressure, volume or temperature. The laws combined form the general gas law:
P x V / T = C
Pressure is given in bar, temperature in Kelvin (gr C = 273.16 and volume in liters. With this formula we can calculate, for example, the extent to which the gas becomes warmer when filling a diving cylinder.
Suppose we fill a 10 liter cylinder with our scubacompressor to 200 bar. Suppose the cylinder is heated up to 57 degrees Celsius by the filling process. We then go diving in water of 7 degrees Celsius. What will then be the pressure in the cylinder?
10 litre x 200 bar/ (273K+57gr Celsius) = 10 x p /(273K+7 gr Celsius)
So much for the gas laws important to diving. However, there is another bouncer, Avogadro’s law. This law shows that the volume of a gas is directly proportional to the number of moles of the gas. An interesting wrap-up to the fourth law that ultimately provided the General Gas Law.
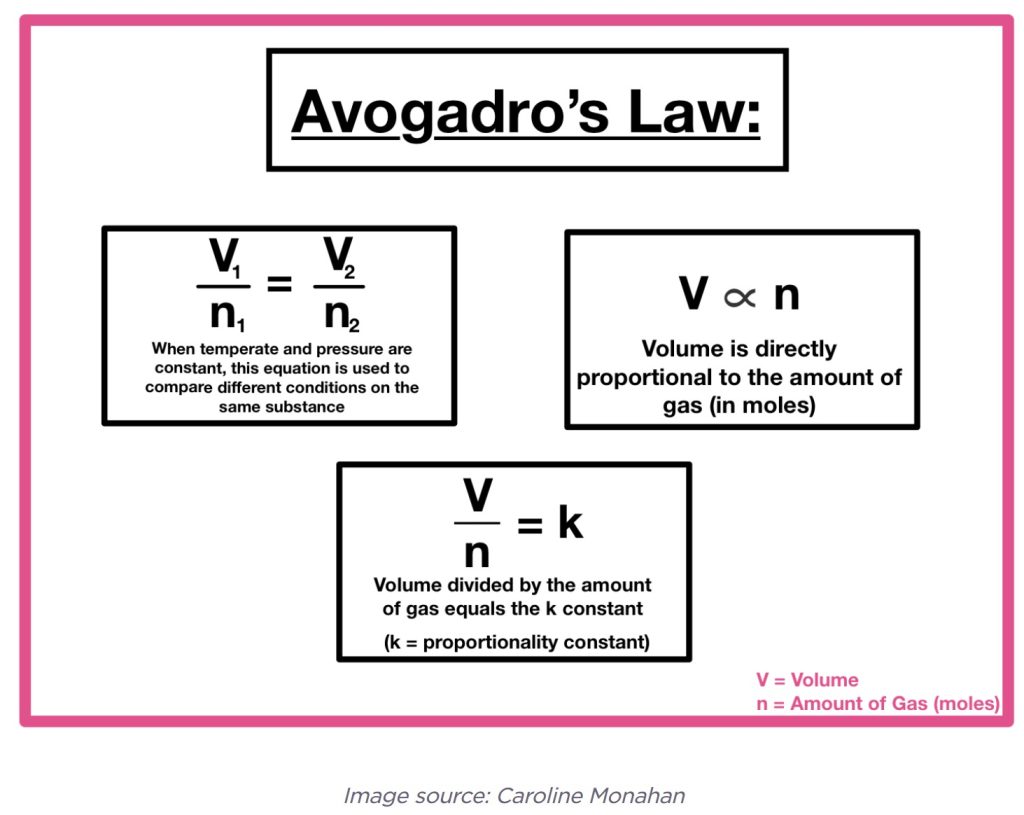
Highly recommended is another look at the calculations with Avogadro’s law on the youtube movie below!

Therebreathersite was founded by Jan Willem Bech in 1999. After a diving career of many years, he decided to start technical diving in 1999. He immediately noticed that at that time there was almost no website that contained the history of closed breathing systems. The start for the website led to a huge collection that offered about 1,300 pages of information until 2019. In 2019, a fresh start was made with the website now freely available online for everyone. Therebreathersite is a source of information for divers, researchers, technicians and students. I hope you enjoy browsing the content!
