How the Electronic Closed circuit Rebreather works
By Jan Willem Bech
January 2023

In an earlier article, we read about closed breathing systems that work with oxygen. One limitation was that these system work with pure oxygen and can therefore be safely dived to a maximum of 6 metres.
Later, semi-closed systems were developed. This involved breathing gas with nitrogen, creating a surplus of gas due to the nitrogen present.
In the early 1950s, the first steps were taken to develop a closed system in which the gas dose was controlled by a computer and a solenoid (injector). Initially, these systems were still very primitive because reliable oxygen sensors did not yet exist. In the years that followed, other techniques were constantly applied, making the ECCR increasingly reliable.
Today, there is a large range of ECCR systems, almost all of which have similar features but most importantly offer the diver long dive times at depths of up to more than 100 metres. The diving devices have CE approval and comply with standards laid down for Europe in a European Standard.
The main difference between a semi-closed system (SCR) and a closed ECCR system lies in the fact that the SCR operates with a fixed fraction of gas, while the ECCR uses a fixed set point of partial oxygen pressure.
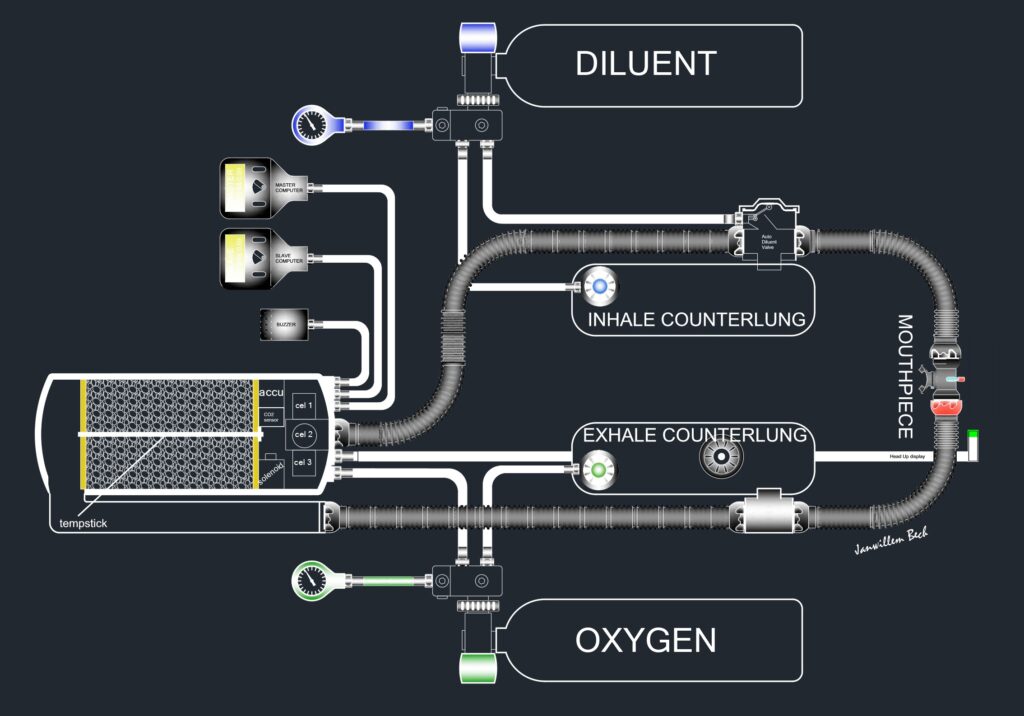
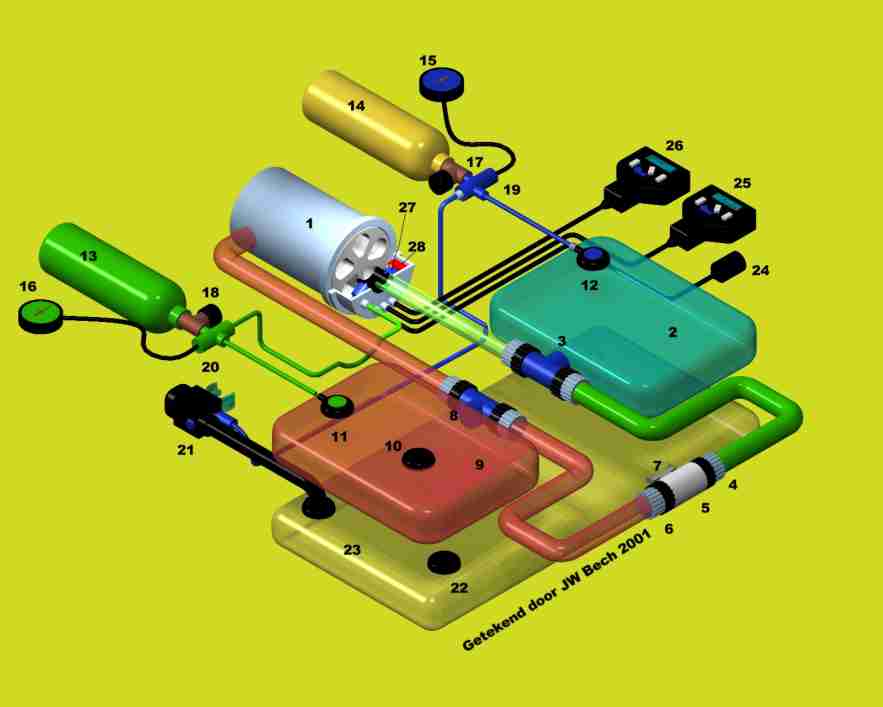
By measuring the partial oxygen level in the unit, the computer can always add enough oxygen from the gas composition to maintain a constant partial oxygen pressure. If a setpoint with a partial pressure of 1.3 bar is chosen, this will be the oxygen pressure over the entire dive. An exception is the first 3 metres. This is because at the surface a setpoint of 1.3 bar will not be achieved. After all, at the surface there is 1 bar and a pressure of 1.3 bar can never be achieved in the loop. Therefore, a second setpoint will often be programmed. This is usually 0.7 bar. At the surface, the electronics will therefore inject oxygen into the loop until it contains 70% oxygen and the setpoint at that point is 0.7 bar pO2. The remaining gas we call the dilution gas in the loop will usually be nitrogen, unless another dilution gas is used. The dilution gas is needed to supplement the setpoint of 1.3 bar with another gas to achieve the pressure at depth. If the diver dives to 50 metres, a dilution gas will be needed there in addition to the 1.3 bar of oxygen to achieve the ambient pressure of 6 bar. If air were to be used as the dilution gas, another problem would arise. Namely, the nitrogen will now form a pressure of 4.7 bar in addition to the oxygen of 1.3 bar and, as we know, nitrogen is strongly narcotic at this pressure. We will therefore have to add a third gas. We often then choose Helium because of its inert properties and availability. So the ECCR diver now dives with an oxygen cylinder with 100% oxygen, and with a trimix mixture which in terms of composition belongs to the intended dive depth. The area between 0 and 3 metres is covered with a lower setpoint. We usually choose 6 metres depth to switch the setpoint from 0.7 to 1.3. Some rebreathers require manual setpoint switching for this, others do it automatically where the ambient pressure initiates this moment. During the descent, the lung volume of the contralungs will be greatly reduced by the ambient pressure. We will need to replenish gas for this. Early ECCR systems required the diver to do this manually by pressing a button that added dilungas to the loop. Modern systems use a so-called ADV, which stands for Automatic Diluent Valve. This valve injects diluent gas by injecting gas into the contralung due to the increase in ambient pressure. This is completely automatic and allows the diver to concentrate on the descent and his setpoint.
One of the great advantages of the Closed Circuit Rebreather (CCR) is that the diver dives with a fixed partial oxygen pressure. During the dive, the oxygen fraction changes with depth. This principle is the exact opposite of the Semi-Closed rebreather. The SCR has a fixed fraction and the oxygen partial pressure changes with depth.
Example: when a CCR diver has a partial pressure of 1.3 bar O2 (setpoint = 1.3), he will inhale 1.3 bar of oxygen at any depth. When diving at a depth of 30 metres, he will inhale a gas with a pressure of 4 bar. If the dilution gas is air, he will inhale 1.3 bar of oxygen and 2.7 bar of nitrogen. The oxygen cells in the rebreather measure the oxygen and add pure oxygen until 1.3 bar is reached. The oxygen in the diluent is included in the 1.3 bar! The diver will inhale a fraction of (1 : 4.0 bar) x 1.3 bar = 0.325. If the oxygen fraction is 0.325, the diver will inhale EAN 32.5. At 50 metres (depth limit with air as diluent), the diver will inhale 6 bar of gas. The mixture is then (1:6.0 bar) x 1.3 = 0.216. The diver breathes EAN 21.6. Here the diver’s gas is similar to air. By this calculation, we see that the fraction changes and the oxygen content of the gas decreases until at 50 metres the breathing gas is comparable to air. If we want to go deeper, we need to replace some of the nitrogen with helium. (When diving with air as a diluent, a new danger arises because if there is a problem with the oxygen supply, it is not possible to flush down the set point).
A closed-circuit rebreather works with setpoints. Setpoints are values chosen by the diver. A setpoint is the chosen value for oxygen partial pressure. We would like to have as high a setpoint as possible, but we are limited by oxygen toxicity. We now know that convulsions can occur in the 1.4 – 1.6 bar oxygen pressure range. Therefore, we choose a lower setpoint of 1.3 or 1.2 bar. Usually, a setpoint of 1.3 bar pO2 is used. The rebreather will constantly inject oxygen until the breathing circuit contains 1.3 bar of oxygen. This is independent of the dive depth. At the surface, however, the 1.3 bar will never be reached because the ambient pressure is 1.0 bar. Should the setpoint at the surface be set to 1.3 bar, the rebreather would continuously add oxygen.
Before diving, the rebreather must be calibrated. There are different techniques for calibrating a CCR. However, it is good practice to calibrate with pure oxygen. If the rebreather can calibrate automatically, usually the mouthpiece is put in the open position, and the computer injects oxygen until there is a stable reading on all 3 cells. This is the reference because the measured voltage of the oxygen cells is relevant for contact with 100% (99.95%) oxygen. High-end systems also calculate with ambient pressure, as this is another factor to make the calibration more accurate.
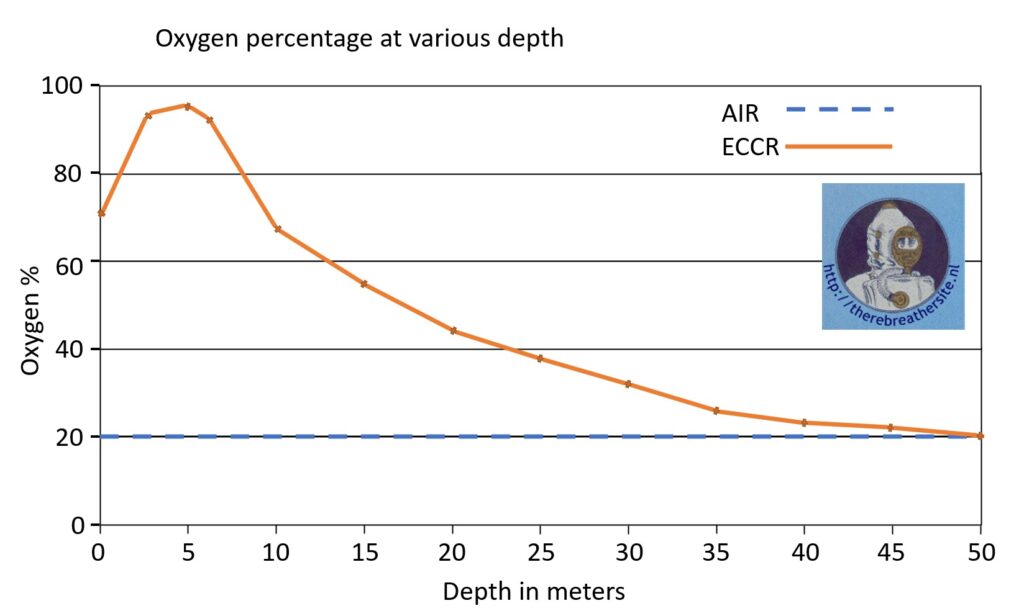
This diagram shows the fraction of oxygen in the breathing air of a closed-circuit rebreather compared to an open-circuit diver. The yellow line shows a decrease in the fraction up to 50 metres. Also visible is the transition from low setpoint to high setpoint at 5 metres.
Gas consumption:
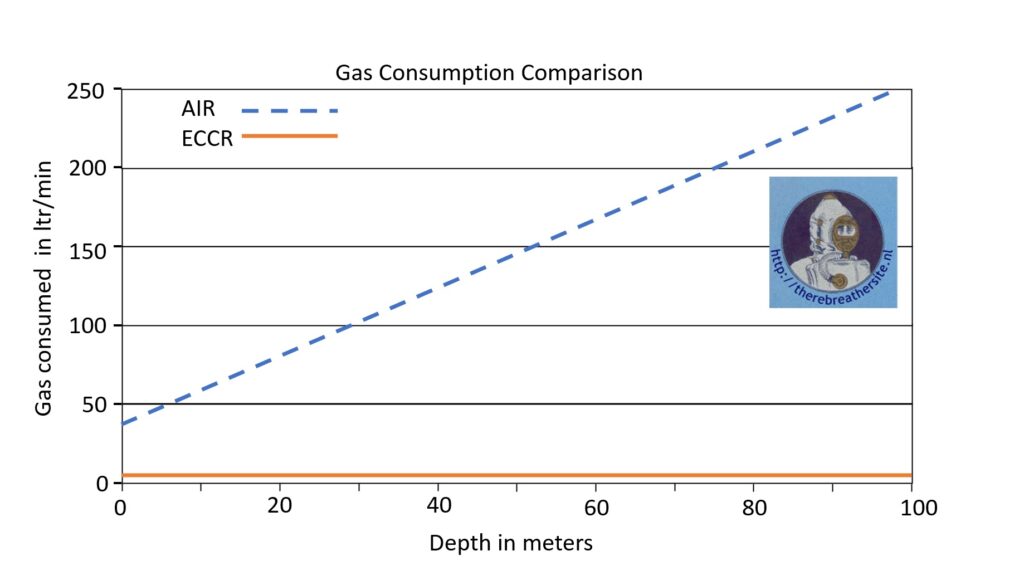
As this rebreather has a closed cycle, the only gas consumed by the diver is oxygen. This consumption is only related to the diver’s metabolic use. Metabolic consumption is related to the energy the diver uses to swim or work. Usually, oxygen consumption is 1 – 1.5 ltr per minute. This is the only gas the diver needs when swimming at the same depth. When he wants to ascend, he needs to inject some gas into his dry suit or wing. When descending, he needs to add some gas to his contralung to maintain a breathing volume. Compared to an open circuit diver, closed circuit diving has 20 times higher gas efficiency! Most interestingly, the CC rebreather diver uses the same amount of gas at any depth, while the open circuit diver’s gas consumption increases with depth!
This table shows the gas consumption of a CC diver versus OC diver. The yellow line is drawn at 1 ltr gas consumption per minute.
This table shows the advantage in decompression requirement. If you compare the nitrogen content of the breathing gas to a depth of 50 metres, the nitrogen content is much lower in closed-circuit diving. So not only is much lower gas consumption achieved, it is also possible to stay down much longer 😉
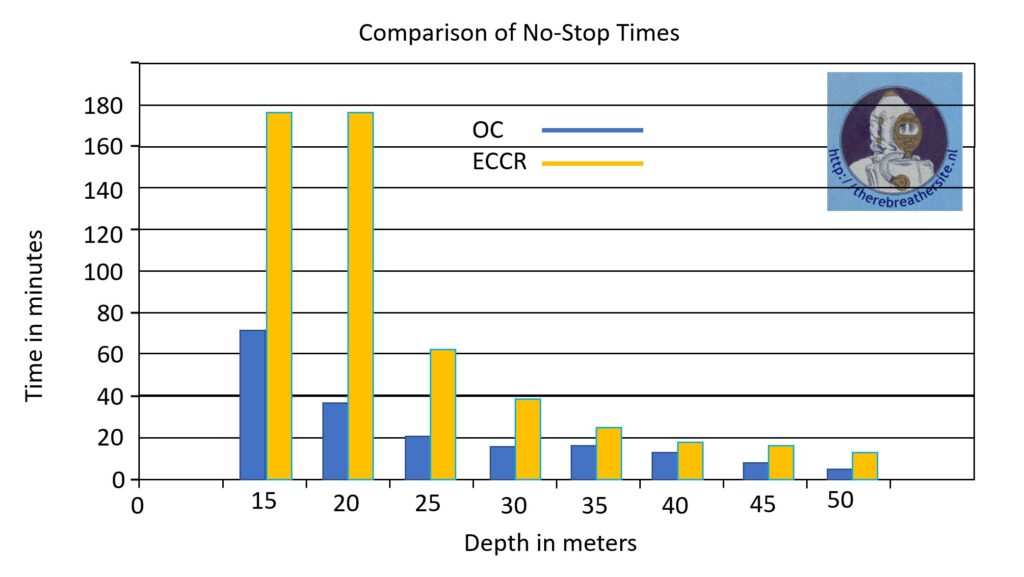
This table clearly shows that diving with the CCR offers the best decompression benefits in the 15-25 metre zone. Fortunately, this is also the most frequently dived zone when we use the rebreather for recreational diving. When we dive deeper than 50 metres, helium is usually added. So we dive with pure oxygen in one bottle and Trimix in the other. We can dive to a depth of 150+ metres and have the advantage of low gas consumption and decompression with high partial oxygen pressures.

Modern CC rebreathers offer real time decompression. Real time decompression means that the system is equipped with a computer that measures the oxygen content and thus knows the nitrogen content to calculate decompression obligations. Also Trimix version can be uses since the content of the makeup gas (diluent) can be programmed into the computer. Decompression is calculated with several algorithm like Buhlmann, VPM or RGBM for example. Alarms can be programmed and several user options are available. Real time decompression has the big advantage that the rebreather controls the setpoint by measuring the oxygen. It is the best way to calculate decompression with the same oxygen cells.

Head up displays (HUD) are more and more popular. Beware that a HUD is an additional device for the diver to have visual control over the rebreather status. It is not a primary source for knowing the pO2!

One of the major dangers of the rebreather is the risk of Hyperoxia. Now in 2023, the temp stick is considered one of the most reliable techniques to monitor the scrubber. However, a temp stick does not measure CO2 but only a moving heat front. As the front approaches the top of the scrubber, the performance of the Sofnolime will decrease. By using intelligent software to project this temperature front, a reasonably reliable estimate can be made of the filtering capacity of a scrubber. In addition to the tempstick, the CO2 sensor is increasingly being used. This gives a warning if too high a value of CO2 is measured. With the arrival of the temp stick in combination with the CO2 sensor, a big step has already been made to monitor the scrubber in terms of operation. In case of a forgotten cartridge, an alarm will go off immediately. So will a forgotten O-ring. Channeling will also trigger an alarm. Unfortunately, developments have not yet reached the point where we have also realised End Tidal CO2 monitoring. Here, the CO2 exhaled by the diver will be measured and the most reliable information can be determined whether the system (and the diver) are functioning correctly.

Rebreathers used in the EU should have CE marking and be build conform EN 14143:2003. A new version will be released the next few years.

CC rebreathers might be equipped with Bail Out Valves (BOV). These BOV’s offer the diver a fast way to switch to open circuit.

The latest generation of CC rebreather also have a “black box”. This technology offers the diver a way to log his dives, and the factory to read out data in case of an accident.

CO2 sensors are often used alongside the temp stick to monitor CO2. Please note that neither the tempstick nor the CO2 monitor actually outputs a CO2 reading. The CO2 sensor only alarms when a CO2 concentration is reached!

Therebreathersite was founded by Jan Willem Bech in 1999. After a diving career of many years, he decided to start technical diving in 1999. He immediately noticed that at that time there was almost no website that contained the history of closed breathing systems. The start for the website led to a huge collection that offered about 1,300 pages of information until 2019. In 2019, a fresh start was made with the website now freely available online for everyone. Therebreathersite is a source of information for divers, researchers, technicians and students. I hope you enjoy browsing the content!
