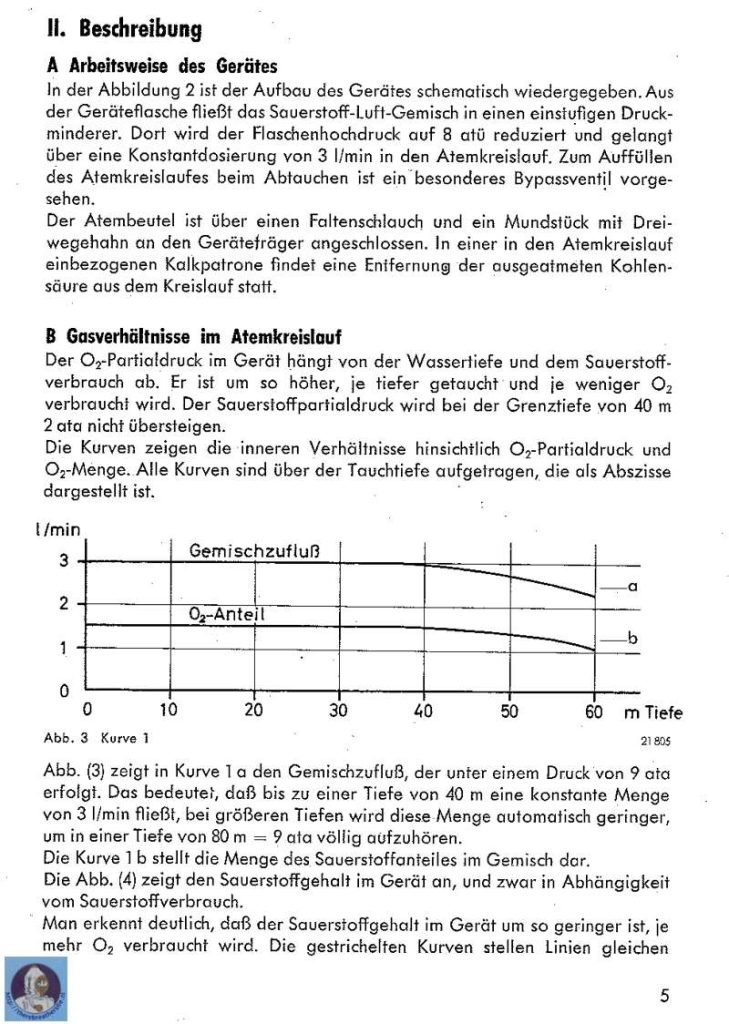
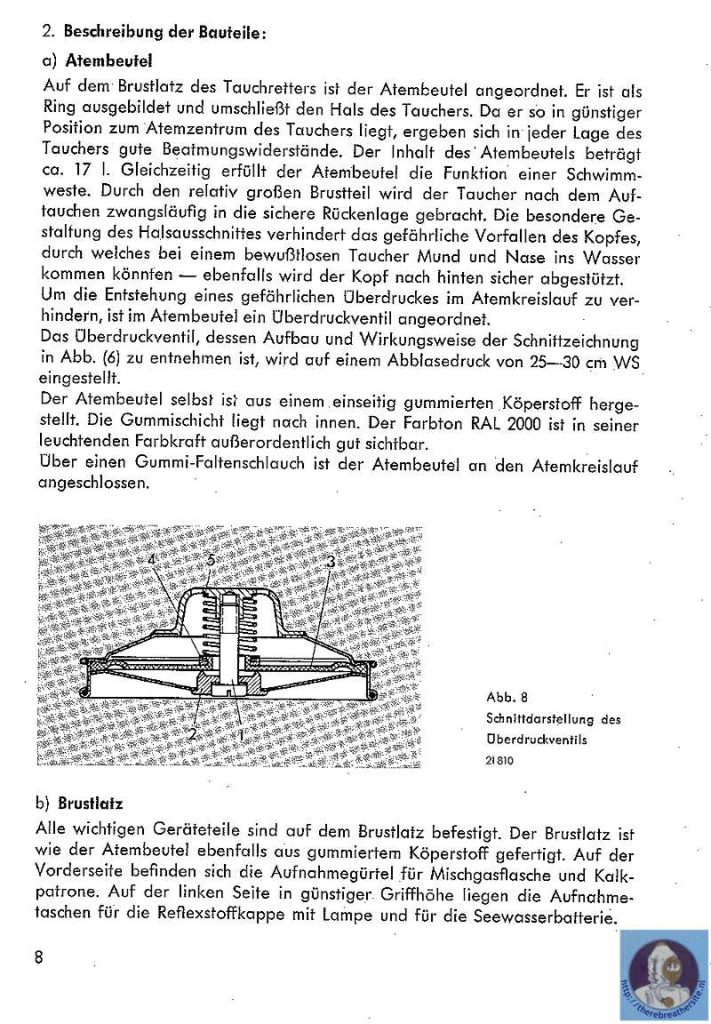
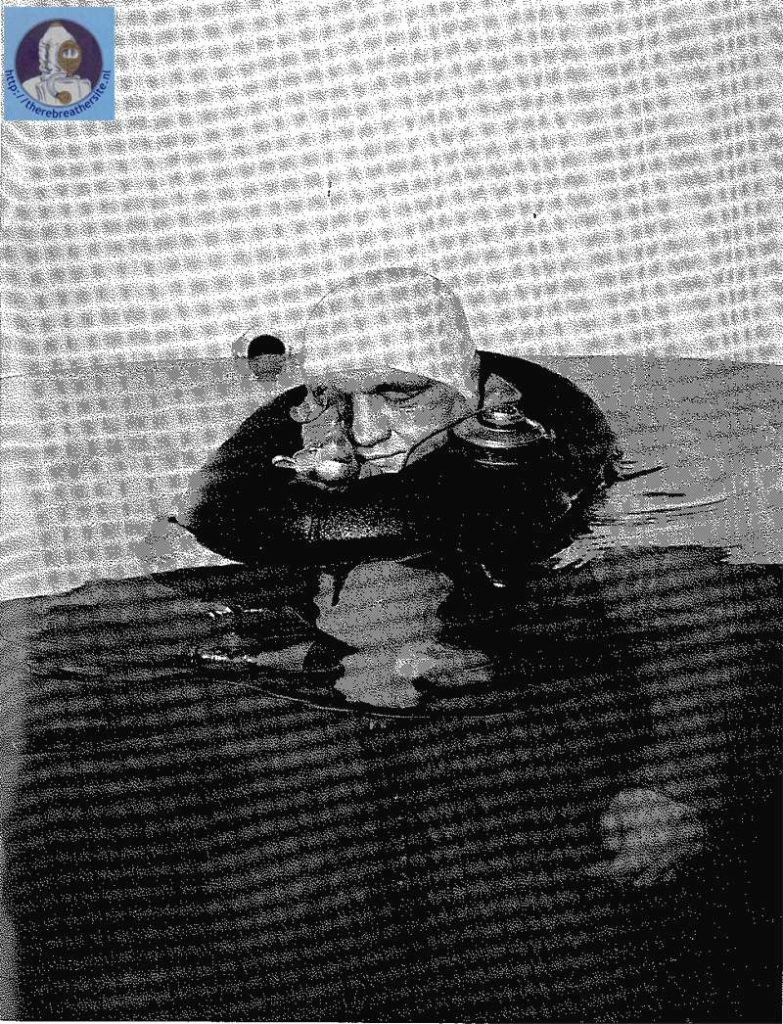
One of the first nitrox tauchretters build in the 60’s

Recently I was able to buy a Dräger Tauchretter Model TR 60. This life saving dive jacket was designed in the 60’s and is a surprising rebreather. One of the most interesting aspects of this rebreather is that in fact it is a semiclosed rebreather! It is well known that using pure oxygen rebreathers to escape from depth introduces a big risk of oxygen toxicity. Semiclosed rebreathers offer a improvement because the breathed gas has a much lower oxygen content. This rebreather uses EAN 50!
To understand how the rebreather works on depth I here made two calculations to show why the partial pressure of oxygen is much lower with a semiclosed rebreather compared tot the standard oxygen rebreathers. The working principle is based on the semi-closed equation:
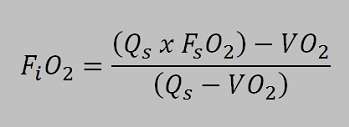
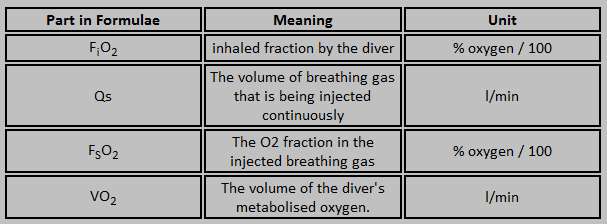
The unit is equipped with a constant mass flow regulator with a gas flow of 3,0 l/min. This flow is constant to a depth of 40 meter. This means that the intermediate pressure is 10 bar on the orifice. The flow will continue to 10 bar and the stops. This will happen o a depth of 90 meters. Becauese the flow is constant to half the IP at 40 meter there will be exact 3,0 ltr/min. Imagine a diver who uses 0,5 ltr oxygen per minute the partial pressure he is breathing can be calculated with the mentioned equation.
| FiO2 = unknown |
| Qs = 3,0 ltr/min |
| FsO2 = 0,5 (EAN 50) |
| VO2 = 0,5 ltr/min |
FiO2 = ((3,0ltr/min x 0,5)-0,5)) / (3,0 ltr/min – 0,5 ltr/min) = 0,4 meaning the fraction of the inhaled gas is 0,4. The gas is EAN 40 breathed at 40 meter the partial pressure of oxygen at depth is 5 bar ambient pressure x 0,4 bar oxygen pressure = 2 bar ! In the 60’s military divers where supposed to be capable of breathing 2 bar oxygen! Fortunately this has been changed, although this unit would only be used in a emergency situation and therefore the 2,0 bar is not so crazy..
When the diver uses more oxygen due to swimming or stress the unit can be used deeper. Imagine a oxygen consumption of 0,8 liter per minute the inspired gas is:
iO2 = ((3,0ltr/min x 0,5)-0,8)) / (3,0 ltr/min – 0,8 ltr/min) = 0,318. When a gas is breathed with a fraction of 0,318 th maximum depth when 2 bar oxygen pressure is allowed is 2bar / 0,318 = 6,28 bar. On a depth of 52,8 meter the rebreather feeds EAN 31,8 resulting in a oxygen pressure of 2bar.
In the following manual you find more details about this very nice unit!




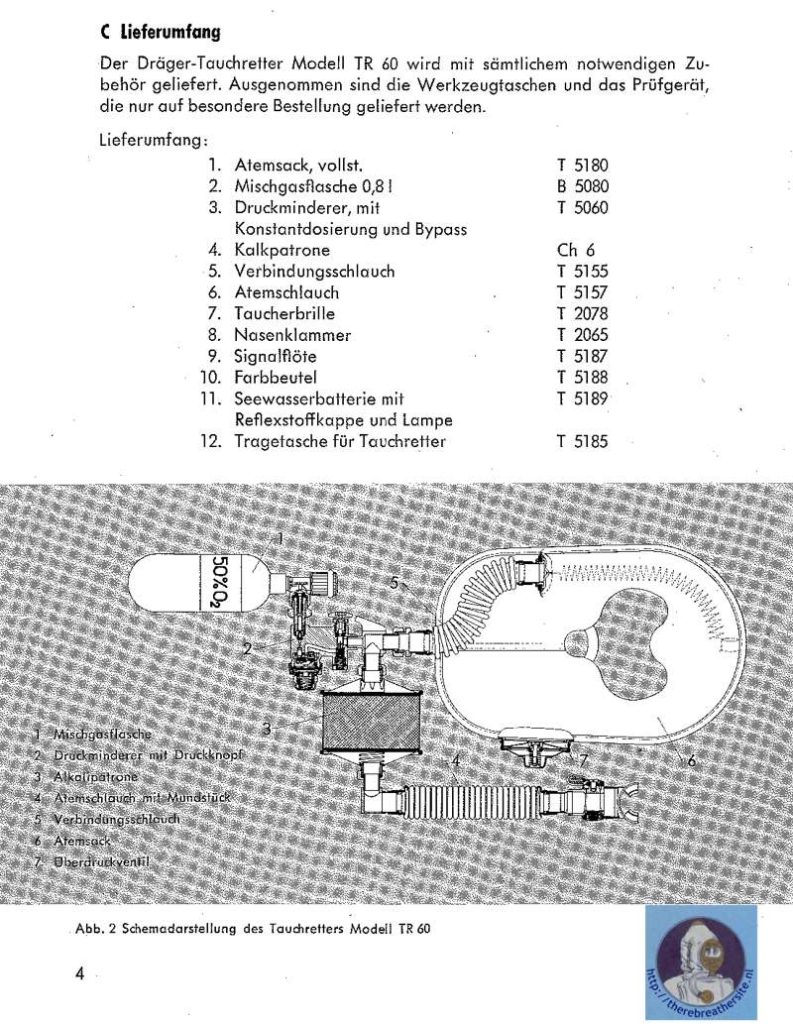
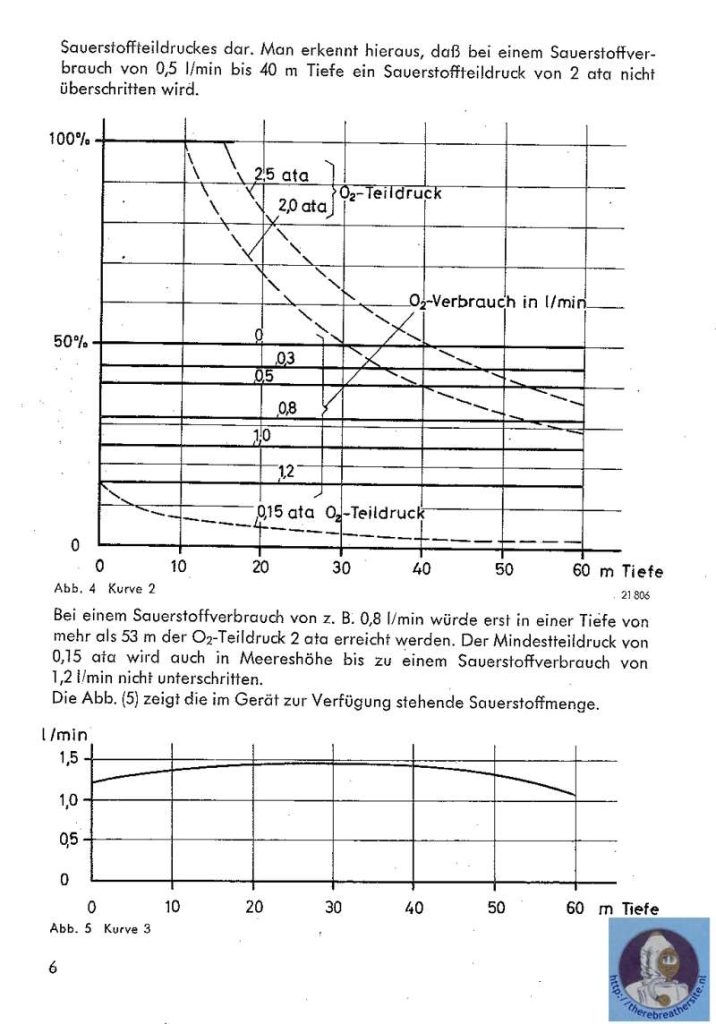







I would like to thank Joe Brandt for his kind cooperation sending me the manual and info about this great unit.
Please check Joe’s website here





































Therebreathersite was founded by Jan Willem Bech in 1999. After a diving career of many years, he decided to start technical diving in 1999. He immediately noticed that at that time there was almost no website that contained the history of closed breathing systems. The start for the website led to a huge collection that offered about 1,300 pages of information until 2019. In 2019, a fresh start was made with the website now freely available online for everyone. Therebreathersite is a source of information for divers, researchers, technicians and students. I hope you enjoy browsing the content!
