Helium
Helium is an important gas for diving because it enables deep diving. It is safer than hydrogen because it is a noble gas and has a weight of 0.17 kg/m3.As the previous section on oxygen and nitrogen showed, these gases pose a problem for the diver at depth. Oxygen because it becomes toxic at a partial pressure of 1.4 – 1.6 bar and nitrogen because it becomes narcotic at a partial pressure of around 3.6 bar. In practice, this means that if we want to dive deeper, we have to add a gas to keep the partial pressures of oxygen and nitrogen as low as possible. For example, if we wanted to dive to a depth of 120 metres, the ambient pressure would be 13 bars. So we need to breathe a gas that is non-toxic and non-narcotic at 13 bar. For that we use Helium! If we want to breathe a maximum of 1.3 bar of oxygen and 2.7 bar (for example, it is a personal choice!) of nitrogen, we need to add another 9 bar of helium to obtain a breathing mixture that can be breathed at that depth. The composition of that gas would then be 1.3/13 x 100 =10% oxygen, 2.7/13 x 100 = 20.7% nitrogen and 9.0/13×100= 69.3% helium. The mixture to be made would then be called TX 10/70, i.e. 10% oxygen and 70% helium. If we were to carry out this dive using an open-circuit breathing system (regulator with bubbles), it would be an expensive dive. This is because Helium is a precious gas that has to be paid for very dearly!
About the Element Helium
Atomic Number: 2
Atomic Weight: 4.002602
Melting Point: 0.95 K (-272.2°C or -458.0°F)
Boiling Point: 4.22 K (-268.93°C or -452.07°F)
Density: 0.0001785 grams per cubic centimeter
Phase at Room Temperature: Gas
Element Classification: Non-metal
Period Number: 1
Group Number: 18
Group Name: Noble Gas
What’s means Helium? Greek god of the sun, Helios.
History and Uses:
Helium, the second most abundant element in the universe, was discovered on the sun before it was found on the earth. Pierre-Jules-César Janssen, a French astronomer, noticed a yellow line in the sun’s spectrum while studying a total solar eclipse in 1868. Sir Norman Lockyer, an English astronomer, realized that this line, with a wavelength of 587.49 nanometers, could not be produced by any element known at the time. It was hypothesized that a new element on the sun was responsible for this mysterious yellow emission. This unknown element was named helium by Lockyer.
The hunt to find helium on earth ended in 1895. Sir William Ramsay, a Scottish chemist, conducted an experiment with a mineral containing uranium called clevite. He exposed the clevite to mineral acids and collected the gases that were produced. He then sent a sample of these gases to two scientists, Lockyer and Sir William Crookes, who were able to identify the helium within it. Two Swedish chemists, Nils Langlet and Per Theodor Cleve, independently found helium in clevite at about the same time as Ramsay.

Helium makes up about 0.0005% of the earth’s atmosphere. This trace amount of helium is not gravitationally bound to the earth and is constantly lost to space. The earth’s atmospheric helium is replaced by the decay of radioactive elements in the earth’s crust. Alpha decay, one type of radioactive decay, produces particles called alpha particles. An alpha particle can become a helium atom once it captures two electrons from its surroundings. This newly formed helium can eventually work its way to the atmosphere through cracks in the crust.
Helium is commercially recovered from natural gas deposits, mostly from Texas, Oklahoma and Kansas. Helium gas is used to inflate blimps, scientific balloons and party balloons. It is used as an inert shield for arc welding, to pressurize the fuel tanks of liquid fueled rockets and in supersonic windtunnels. Helium is combined with oxygen to create a nitrogen free atmosphere for deep sea divers so that they will not suffer from a condition known as nitrogen narcosis. Liquid helium is an important cryogenic material and is used to study superconductivity and to create superconductive magnets. The Department of Energy’s Jefferson Lab uses large amounts of liquid helium to operate its superconductive electron accelerator.
Helium is an inert gas and does not easily combine with other elements. There are no known compounds that contain helium, although attempts are being made to produce helium diflouride (HeF2).
Data thanks to: JLAB science education
Helium: Sources and Uses
Helium is thought to be the most abundant element in the universe , as it the product of the fusion reaction that happens in the heart of stars. On Earth, however helium is exceedingly rare and comprises only 5.2 ppm of Earth’s atmosphere. Most of the helium on Earth comes from radiological sources. As elements like uranium and thorium decay deep underground into thorium and radium, respectively, α-particles consisting of two protons and two neutrons are released and trapped. As these α-particles pick up electrons from their environment they become stable helium atoms.
U238→Th234+α; U235→Th231+α; Th232→Ra228+α
The major sources for terrestrial helium are natural gas deposits that can contain up to 2.7% helium. Because helium is a noble gas with a full electron shell, it is extremely unlikely to react with other chemicals, and once it reaches the surface, rapidly rises to the highest levels of the atmosphere. Over most of the planet, the Earth’s magnetic field lines are able to retain the vast majority of our atmosphere; however over the poles, these field lines trail off due to the solar wind allowing lighter atoms like hydrogen and helium to escape.
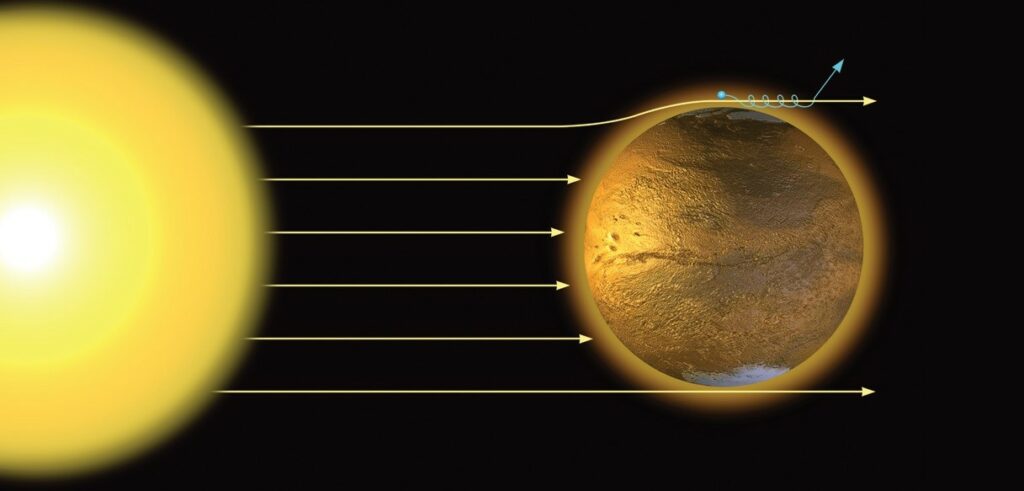
This polar wind is the cause of almost all of Earth’s lost helium at a rate of 1600 metric tons per year. For many years and, in many cases still today, radiogenic helium was wasted as a byproduct of the natural gas extraction process. It was only during World War I that the federal government began to recognize the usefulness of the noble gas for potential military applications including dirigible flight. In 1917, Congress authorized the Federal Helium Program and started processing helium in its facility in Texas. The Bureau of Land Management (BLM) under the Department of the Interior extracts and refines helium from the Hugoton shale field in Oklahoma and pipes it into the natural caves of the Bush Dome Reservoir in Amarillo, TX, that are used as the storage facility. As of 2013, 35% and 70% of the world’s helium supply came from the Federal Helium Reserve in specific and the United States in general.
While helium has moved far beyond its original military dirigible use, today it is still used for flight in certain rockets and weather balloons, and for the welding of advanced materials like titanium needed for such crafts using helium arc welding. It is used extensively by deep sea divers to avoid the bends. Most importantly for our purposes it is used in its liquid state at 4K (-452F) to perform cutting edge scientific experiments probing the structure of matter across physics, chemistry, materials science, biology, and medicine.
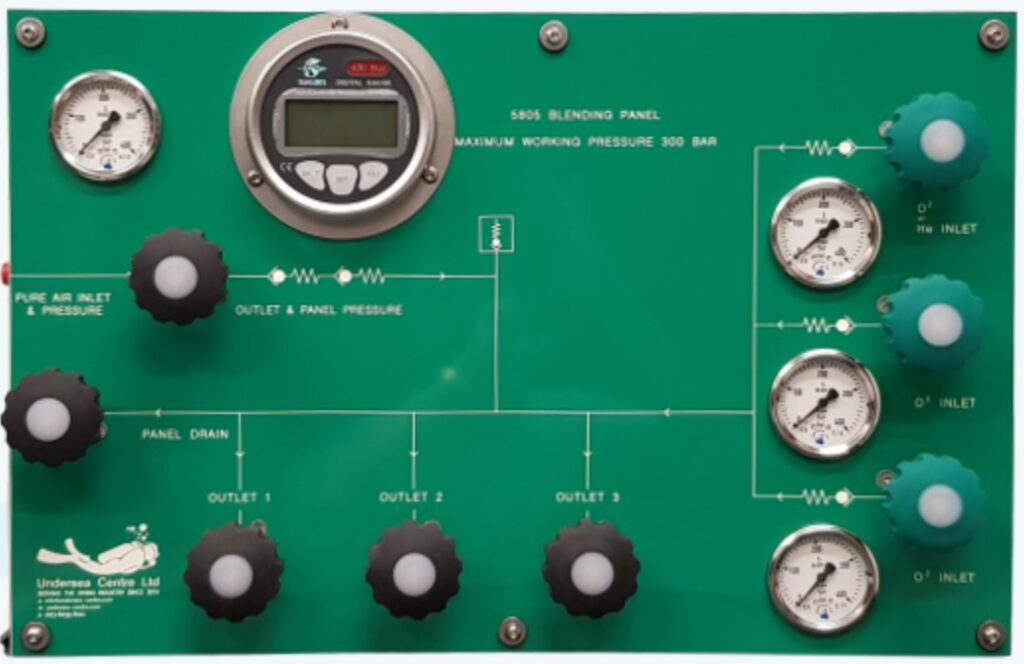
Helium production in the World:
Companies involved in Helium production and distribution:
Airgas Air Liquid Linde Messer Group Air Products Gazprom Gulf Cryo Matheson Tri-Gas Exxon Praxair Buzwair Badger Midstream Iceblick RasGas Taiyo Nippon Sanso PGNiG NexAir LLC Qatargas Operating Company Limited Renergen Weil Group Iwatani Corporation
Further Background
During the early 1900s, the development of lighter-than-air blimps and dirigibles relied almost entirely on hydrogen to provide lift, even though it was highly flammable. During World War I, the United States government realized that non-flammable helium was superior to hydrogen and declared it a critical war material. Production was tightly controlled, and exports were curtailed. In 1925, the United States passed the first Helium Conservation Act which prohibited the sale of helium to nongovernmental users. It wasn’t until 1937, when the hydrogen-filled dirigible Hindenburg exploded while landing at Lakehurst, New Jersey, that the restrictions were lifted and helium replaced hydrogen for commercial lighter-than-air ships.
During World War II, helium became a critical war material again. One of its more unusual uses was to inflate the tires on long-range bomber aircraft. The lighter weight of helium allowed the plane to carry 154 lb (70 kg) of extra fuel for an extended range.
After the war, demand for helium grew so rapidly that the government imposed the Helium Act Amendments in 1960 to purchase and store the gas for future use. By 1971, the demand had leveled off and the helium storage program was canceled. A few years later, the government started storing helium again. As of 1993, there were about 35 billion cubic feet (1.0 billion cubic meters) of helium in government storage.
Today, the majority of the helium-bearing natural gas sources are within the United States. Canada, Poland, Russia and a few other countries also have significant sources.
Raw Materials
Helium is generated underground by the radioactive decay of heavy elements such as uranium and thorium. Part of the radiation from these elements consists of alpha particles, which form the nuclei of helium atoms. Some of this helium finds its way to the surface and enters the atmosphere, where it quickly rises and escapes into space. The rest becomes trapped under impermeable layers of rock and mixes with the natural gases that form there. The amount of helium found in various natural gas deposits varies from almost zero to as high as 4% by volume. Only about one-tenth of the working natural gas fields have economically viable concentrations of helium greater than 0.4%.
Helium can also be produced by liquefying air and separating the component gases. The production costs for this method are high, and the amount of helium contained in air is very low. Although this method is often used to produce other gases, like nitrogen and oxygen, it is rarely used to produce helium.
The Manufacturing Process
Helium is usually produced as a byproduct of natural gas processing. Natural gas contains methane and other hydrocarbons, which are the principal sources of heat energy when natural gas is burned. Most natural gas deposits also contain smaller quantities of nitrogen, water vapor, carbon dioxide, helium, and other non-combustible materials, which lower the potential heat energy of the gas. In order to produce natural gas with an acceptable level of heat energy, these impurities must be removed. This process is called upgrading.
There are several methods used to upgrade natural gas. When the gas contains more than about 0.4% helium by volume, a cryogenic distillation method is often used in order ta wayr the helium content. Once the helium has been separated from the natural gas, it undergoes further refining ta way it to 99.99+% purity for commercial use.
Here is a typical sequence of operations for extracting and processing helium.
Pretreating
Because this method utilizes an extremely cold cryogenic section as part of the process, all impurities that might solidify—such as water vapor, carbon dioxide, and certain heavy hydrocarbons—must first be removed from the natural gas in a pretreatment process to prevent them from plugging the cryogenic piping.
1 The natural gas is pressurized to about 800 psi (5.5 Mpa or 54 atm). It then flows into a scrubber where it is subjected to a spray of monoethanolamine, which absorbs the carbon dioxide and carries ta way.
2 The gas stream passes through a molecular sieve, which strips the larger water vapor molecules from the stream while letting the smaller gas molecules pass. The water is back-flushed out of the sieve and removed.
3 Any heavy hydrocarbons in the gas stream are collected on the surfaces of a bed of activated carbon as the gas passes through it. Periodically the activated carbon is recharged. The gas stream now contains mostly methane and nitrogen, with small amounts of helium, hydrogen, and neon.
Separating
Natural gas is separated into its major components through a distillation process known as fractional distillation. Sometimes this name is shortened to fractionation, and the vertical structures used to perform this separation are called fractionating columns. In the fractional distillation process, the nitrogen and methane are separated in two stages, leaving a mixture of gases containing a high percentage of helium. At each stage the level of concentration, or fraction, of each component is increased until the separation is complete. In the natural gas
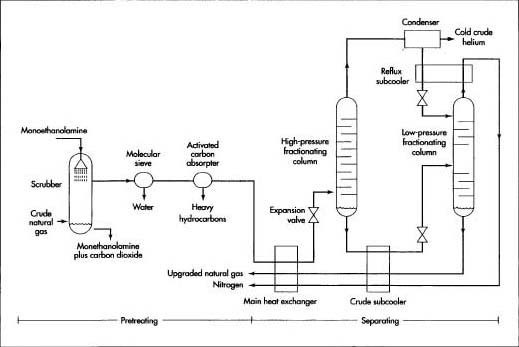
All impurities that might solidify and clog the cryogenic piping is removed from the natural gas in a pretreatment process. After pretreatment, the natural gas components are separated in a process called fractional distillation.
industry, this process is sometimes called nitrogen rejection, since its primary function is to remove excess quantities of nitrogen from the natural gas.
4 The gas stream passes through one side of a plate fin heat exchanger while very cold methane and nitrogen from the cryogenic section pass through the other side. The incoming gas stream is cooled, while the methane and nitrogen are warmed.
5 The gas stream then passes through an expansion valve, which allows the gas to expand rapidly while the pressure drops to about 145-360 psi (1.0-2.5 MPa or 10-25 atm). This rapid expansion cools the gas stream to the point where the methane starts to liquefy.
6 The gas stream—now part liquid and part gas—enters the base of the high-pressure fractionating column. As the gas works its way up through the internal baffles in the column, it loses additional heat. The methane continues to liquefy, forming a methane-rich mixture in the bottom of the column while most of the nitrogen and other gases flow to the top.
7 The liquid methane mixture, called crude methane, is drawn out of the bottom of the high-pressure column and is cooled further in the crude subcooler. It then passes through a second expansion valve, which drops the pressure to about 22 psi (150 kPa or 1.5 atm) before it enters the low-pressure fractionating column. As the liquid methane works its way down the column, most of the remaining nitrogen is separated, leaving a liquid that is no more than about 4% nitrogen and the balance methane. This liquid is pumped off, warmed, and evaporated to become upgraded natural gas. The gaseous nitrogen is piped off the top of the low-pressure column and is either vented or captured for further processing.
8 Meanwhile, the gases from the top of the high-pressure column are cooled in a
condenser. Much of the nitrogen condenses into a vapor and is fed into the top of the low-pressure column. The remaining gas is called crude helium. It contains about 50-70% helium, 1-3% unliquefied methane, small quantities of hydrogen and neon, and the balance nitrogen.
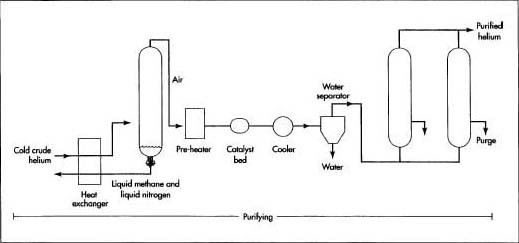
Purifying
Crude helium must be further purified to remove most of the other materials. This is usually a multi-stage process involving several different separation methods depending on the purity of the crude helium and the intended application of the final product.
9 The crude helium is first cooled to about -315° F (-193° C). At this temperature, most of the nitrogen and methane condense into a liquid and are drained off. The remaining gas mixture is now about 90% pure helium.
10 Air is added to the gas mixture to provide oxygen. The gas is warmed in a preheater and then it passes over a catalyst, which causes most of the hydrogen in the mixture to react with the oxygen in the air and form water vapor. The gas is then cooled, and the water vapor condenses and is drained off.
11 The gas mixture enters a pressure swing adsorption (PSA) unit consisting of several adsorption vessels operating in parallel. Within each vessel are thousands of particles filled with tiny pores. As the gas mixture passes through these particles under pressure, certain gases are trapped within the particle pores. The pressure is then decreased and the flow of gas is reversed to purge the trapped gases. This cycle is repeated after a few seconds or few minutes, depending on the size of the vessels and the concentration of gases. This method removes most of the remaining water vapor, nitrogen, and methane from the gas mixture. The helium is now about 99.99% pure.
Distributing
Helium is distributed either as a gas at normal temperatures or as a liquid at very low temperatures. Gaseous helium is distributed in forged steel or aluminum alloy cylinders at pressures in the range of 900-6,000 psi (6-41 MPa or 60-410 atm). Bulk quantities of liquid helium are distributed in insulated containers with capacities up to about 14,800 gallons (56,000 liters).
12 If the helium is to be liquefied, or if higher purity is required, the neon and any trace impurities are removed by passing the gas over a bed of activated carbon in a cryogenic adsorber operating at about -423° F (-253° C). Purity levels of 99.999% or better can be achieved with this final step.
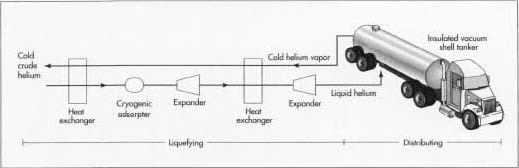
cryogenic adsorber operating at about -423° F (-253° C). Purity levels of 99.999% or better can be achieved with this final step.
13 The helium is then piped into the liquefier, where it passes through a series of heat exchangers and expanders. As it is progressively cooled and expanded, its temperature drops to about -452° F (-269° C) and it liquefies.
14 Large quantities of liquid helium are usually shipped in unvented, pressurized containers. If the shipment is within the continental United States, shipping time is usually less than a week. In those cases, the liquid helium is placed in large, insulated tank trailers pulled by truck tractors. The tank body is constructed of two shells with a vacuum space between the inner and outer shell to retard heat loss. Within the vacuum space, multiple layers of reflective foil further halt heat flow from the outside. For extended shipments overseas, the helium is placed in special shipping containers. In addition to a vacuum space to provide insulation, these containers also have a second shell filled with liquid nitrogen to absorb heat from the outside. As heat is absorbed, the liquid nitrogen boils off and is vented. Article from madehow, Read more: http://www.madehow.com


Therebreathersite was founded by Jan Willem Bech in 1999. After a diving career of many years, he decided to start technical diving in 1999. He immediately noticed that at that time there was almost no website that contained the history of closed breathing systems. The start for the website led to a huge collection that offered about 1,300 pages of information until 2019. In 2019, a fresh start was made with the website now freely available online for everyone. Therebreathersite is a source of information for divers, researchers, technicians and students. I hope you enjoy browsing the content!
